CAZypedia needs your help! We have many unassigned GH, PL, CE, AA, GT, and CBM pages in need of Authors and Responsible Curators.
Scientists at all career stages, including students, are welcome to contribute to CAZypedia. Read more here, and in the 10th anniversary article in Glycobiology.
New to the CAZy classification? Read this first.
*
Consider attending the 15th Carbohydrate Bioengineering Meeting in Ghent, 5-8 May 2024.
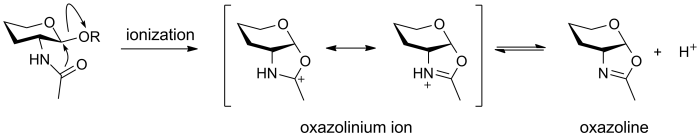
Oxazolinium ion
This page has been approved by the Responsible Curator as essentially complete. CAZypedia is a living document, so further improvement of this page is still possible. If you would like to suggest an addition or correction, please contact the page's Responsible Curator directly by e-mail.
- Author: Spencer Williams
- Responsible Curator: Spencer Williams
An oxazolinium ion is the species that is formed by anchimeric assistence in the glycosidic bond-cleaving reaction of a 2-deoxy-2-acetamido glycoside [1]. Solution studies have shown that the 2-acetamido N-acetyl group enhances spontaneous hydrolysis, of methyl 2-acetamido-2-deoxy-α-D-glucopyranoside relative to methyl α-D-glucopyranoside, by more than 1000-fold [2]. An oxazolinium ion can be deprotonated to give an oxazoline, which is a stable and isolable species. Oxazolinium ions are common intermediates in the neighboring group participation mechanism of glycoside hydrolases, including glycoside hydrolases from families GH18, GH20, GH25, GH56, GH84, and GH85.
Anchimeric assistance can occur with modified 2-acetamido groups, such as 2-glycolylamido groups, affording hydroxymethyl substituted oxazolinium ion intermediates, such as that demonstrated for the glycoside hydrolases of GH84 [3].
See also
References
-
Tews, I., Terwisscha van Scheltinga, A.C., Perrakis, A., Wilson, K.S., and Dijkstra, B.W. J. Am. Chem. Soc. 1997, 119, 7954-7959.
-
Piszkiewicz, D.; Bruice, T. C. J. Am. Chem. Soc. 1968, 90, 5844-5848.
- Macauley MS, Chan J, Zandberg WF, He Y, Whitworth GE, Stubbs KA, Yuzwa SA, Bennet AJ, Varki A, Davies GJ, and Vocadlo DJ. (2012). Metabolism of vertebrate amino sugars with N-glycolyl groups: intracellular β-O-linked N-glycolylglucosamine (GlcNGc), UDP-GlcNGc, and the biochemical and structural rationale for the substrate tolerance of β-O-linked β-N-acetylglucosaminidase. J Biol Chem. 2012;287(34):28882-97. DOI:10.1074/jbc.M112.363721 |