CAZypedia needs your help! We have many unassigned GH, PL, CE, AA, GT, and CBM pages in need of Authors and Responsible Curators.
Scientists at all career stages, including students, are welcome to contribute to CAZypedia. Read more here, and in the 10th anniversary article in Glycobiology.
New to the CAZy classification? Read this first.
*
Consider attending the 15th Carbohydrate Bioengineering Meeting in Ghent, 5-8 May 2024.
Glycoside Hydrolase Family 136
This page is currently under construction. This means that the Responsible Curator has deemed that the page's content is not quite up to CAZypedia's standards for full public consumption. All information should be considered to be under revision and may be subject to major changes.
- Author: ^^^Chihaya Yamada^^^
- Responsible Curator: ^^^Shinya Fushinobu^^^
| Glycoside Hydrolase Family GH136 | |
| Clan | GH-N |
| Mechanism | retaining |
| Active site residues | Asp |
| CAZy DB link | |
| http://www.cazy.org/GH136.html | |
Substrate specificities
This family of glycoside hydrolases contains lacto-N-biosidase, as demonstrated for LnbX from Bifidobacterium longum JCM 1217 [1]. LnbX liberates Galβ1-3GlcNAc(lacto-N-biose I, LNB) and lactose from lacto-N-tetraose, the main component of human milk oligosaccharides. It hydrolyzed the linkage GlcNAcβ1-3Gal in lacto-N-hexaose, lacto-N-fucopentaose I, and sialyllacto-N-tetraose a of human milk oligosaccharides as substrate of LnbX in the GH136. In addition, LnbX liberates Galβ1-3GalNAc (GNB) from the sugar chains of globo- and ganglio-series glycosphingolipids [2].
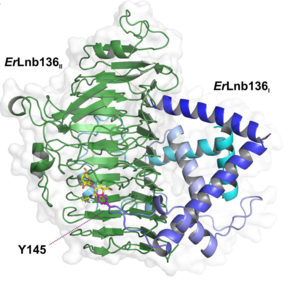
GH136 lacto-N-biosidase required neighboring chaperon gene for folding. Rarely, chaperone-like gene fused to lacto-N-biosidase gene in case of ErLnb136I and ErLnb136IIfrom Eubacterium ramulus [3].
Kinetics and Mechanism
LnbX hydrolyzes the glycosidic linkage via a retaining mechanism involving a Grotthuss proton relay.
Catalytic Residues
The nucleophile is Asp418. The catalytic acid/base is Asp411 via water molecule.
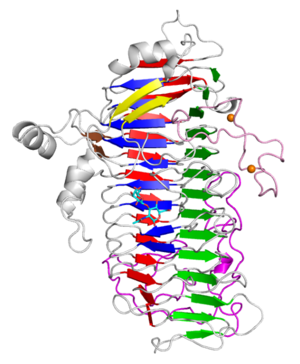
Three-dimensional structures
The X-ray crystal structure of the catalytic domain, LnbXc(31-625) revealed a right-handed β helix fold that is usually shared by polysaccharide active enzymes. Three forms, ligand free at 2.36 Å resolution (PDB ID 5GQC), LNB complex at 1.82 Å (PDB ID 5GQF), and GNB complex at 2.70 Å (PDB ID 5GQG) were determined [4]. The X-ray crystal structure of ErGH136 in complex with LNB (PDB ID 6KQT) revealed the N-terminal domain (ErLnb136I, from AA 7-224) consists of 8 α-helices (α1-α8) and Y145 of the α6-α7 loop positioned near the active site [3].
Family Firsts
- First stereochemistry determination
- Content is to be added here.
- First catalytic nucleophile identification
- Content is to be added here.
- First general acid/base residue identification
- Content is to be added here.
- First 3-D structure
- Content is to be added here.
References
- Sakurama H, Kiyohara M, Wada J, Honda Y, Yamaguchi M, Fukiya S, Yokota A, Ashida H, Kumagai H, Kitaoka M, Yamamoto K, and Katayama T. (2013). Lacto-N-biosidase encoded by a novel gene of Bifidobacterium longum subspecies longum shows unique substrate specificity and requires a designated chaperone for its active expression. J Biol Chem. 2013;288(35):25194-25206. DOI:10.1074/jbc.M113.484733 |
- Gotoh A, Katoh T, Sugiyama Y, Kurihara S, Honda Y, Sakurama H, Kambe T, Ashida H, Kitaoka M, Yamamoto K, and Katayama T. (2015). Novel substrate specificities of two lacto-N-biosidases towards β-linked galacto-N-biose-containing oligosaccharides of globo H, Gb5, and GA1. Carbohydr Res. 2015;408:18-24. DOI:10.1016/j.carres.2015.03.005 |
- Pichler MJ, Yamada C, Shuoker B, Alvarez-Silva C, Gotoh A, Leth ML, Schoof E, Katoh T, Sakanaka M, Katayama T, Jin C, Karlsson NG, Arumugam M, Fushinobu S, and Abou Hachem M. (2020). Butyrate producing colonic Clostridiales metabolise human milk oligosaccharides and cross feed on mucin via conserved pathways. Nat Commun. 2020;11(1):3285. DOI:10.1038/s41467-020-17075-x |
- Yamada C, Gotoh A, Sakanaka M, Hattie M, Stubbs KA, Katayama-Ikegami A, Hirose J, Kurihara S, Arakawa T, Kitaoka M, Okuda S, Katayama T, and Fushinobu S. (2017). Molecular Insight into Evolution of Symbiosis between Breast-Fed Infants and a Member of the Human Gut Microbiome Bifidobacterium longum. Cell Chem Biol. 2017;24(4):515-524.e5. DOI:10.1016/j.chembiol.2017.03.012 |