CAZypedia needs your help! We have many unassigned GH, PL, CE, AA, GT, and CBM pages in need of Authors and Responsible Curators.
Scientists at all career stages, including students, are welcome to contribute to CAZypedia. Read more here, and in the 10th anniversary article in Glycobiology.
New to the CAZy classification? Read this first.
*
Consider attending the 15th Carbohydrate Bioengineering Meeting in Ghent, 5-8 May 2024.
Difference between revisions of "Auxiliary Activity Family 10"
| Line 30: | Line 30: | ||
== Substrate specificities == | == Substrate specificities == | ||
[[Image:CBP21_binding_chitin_modeled.jpg|thumb|right|400px|'''Figure 1. Hypothetical representation of the interaction between CBP21 and chitin (side view, left; top view, right) that highlights how the flat surface of CBP21 might fit the flat surface of a β-chitin crystal.''' Please note that this complex has not been determined by direct experimentation nor computational molecular modelling. The surfaces of residues known to interact with chitin <cite>Vaaje-Kolstad2005-1</cite> are coloured magenta and the side chains of these residues are shown. In the side view some of the magenta surface is hidden by the white surface of other residues. This picture has been adapted from <cite>Horn2012</cite>.]] | [[Image:CBP21_binding_chitin_modeled.jpg|thumb|right|400px|'''Figure 1. Hypothetical representation of the interaction between CBP21 and chitin (side view, left; top view, right) that highlights how the flat surface of CBP21 might fit the flat surface of a β-chitin crystal.''' Please note that this complex has not been determined by direct experimentation nor computational molecular modelling. The surfaces of residues known to interact with chitin <cite>Vaaje-Kolstad2005-1</cite> are coloured magenta and the side chains of these residues are shown. In the side view some of the magenta surface is hidden by the white surface of other residues. This picture has been adapted from <cite>Horn2012</cite>.]] | ||
| − | Members of the AA10 family of lytic polysaccharide monooxygenases were previously classified as [[Carbohydrate Binding Module Family 33]] members). They are known to cleave chitin <cite>Vaaje-Kolstad2010 Vaaje-Kolstad2012 Aachmann2012</cite> and cellulose <cite>Forsberg2011 Forsberg 2014</cite>. AA10 LPMOs are closely related to LPMOs in families AA11 and AA13, known to cleave chitin and starch, respectively. AA10 modules often occur in combination with additional modules, in particular [[carbohydrate-binding modules]] (CBMs), but also catalytic domains such as GH18 chitinases <cite>Horn2012</cite>. The CBMs contribute to substrate-binding and may also affect operational stability of the LPMO <cite>Forsbergb2014 Forsberg2018</cite>. | + | Members of the AA10 family of lytic polysaccharide monooxygenases were previously classified as [[Carbohydrate Binding Module Family 33]] members). They are known to cleave chitin <cite>Vaaje-Kolstad2010 Vaaje-Kolstad2012 Aachmann2012</cite> and cellulose <cite>Forsberg2011 Forsberg 2014</cite>. Most characterized AA10 LPMO oxidize the C1 of the scissile glycosidic bond, but some also oxidize C4 <cite>Forsberg2014 Forsberg2018</cite>. AA10 LPMOs are closely related to LPMOs in families AA11 and AA13, known to cleave chitin and starch, respectively. AA10 modules often occur in combination with additional modules, in particular [[carbohydrate-binding modules]] (CBMs), but also catalytic domains such as GH18 chitinases <cite>Horn2012</cite>. The CBMs contribute to substrate-binding and may also affect operational stability of the LPMO <cite>Forsbergb2014 Forsberg2018</cite>. |
| − | Before proteins belonging to AA10 were identified as enzymes, they were generally known as chitin binding proteins (CBPs). The reason for this was that most AA10s studied had been identified in chitinolytic systems such as that of ''Serratia marcescens'' <cite>Fuchs1986, Suzuki1998</cite>, several ''Streptomyces'' species <cite>Zeltins1997 Kolbe1998 Saito2001 Chu2001</cite>, ''Vibrio cholerae'' <cite>Wong2012</cite>, ''Pseudomonas aeruginosa'' <cite>Folders2000</cite> and ''Lacotococcus lactis'' <cite>Vaaje-Kolstad2009</cite>. Upon their characterization no other function than substrate binding could be identified, thus the name "chitin binding protein" was coined. Substrates identified | + | Before proteins belonging to AA10 were identified as enzymes, they were generally known as chitin binding proteins (CBPs). The reason for this was that most AA10s studied had been identified in chitinolytic systems such as that of ''Serratia marcescens'' <cite>Fuchs1986, Suzuki1998</cite>, several ''Streptomyces'' species <cite>Zeltins1997 Kolbe1998 Saito2001</cite>, ''Bacillus amyloliquefaciens'' <cite>Chu2001</cite>,''Vibrio cholerae'' <cite>Wong2012</cite>, ''Pseudomonas aeruginosa'' <cite>Folders2000</cite> and ''Lacotococcus lactis'' <cite>Vaaje-Kolstad2009</cite>. Upon their characterization no other function than substrate binding could be identified, thus the name "chitin binding protein" was coined. Substrates and potential substrates identified by binding studies include alpha-chitin <cite>Kolbe1998 Zeltins1997</cite>, beta-chitin <cite>Suzuki1998 Vaaje-Kolstad2005-1</cite>, both the alpha- and beta-chitin allomorphs <cite>Chu2001 Vaaje-Kolstad2009 Vaaje-Kolstad2012</cite> chitosan <cite>Saito2001</cite>, cellulose <cite>Walter2008 Forsberg2011</cite> and even bacterial and eptihelial cell surfaces where the binding interaction substrate has been suggested to be GlcNAc containing glycoproteins or proteoglycans <cite>Sanches2011 Wong2012</cite>. It should be noted that studies on AA10s prior to their identification as copper-dependent metalloenzymes were conducted in the absence of Cu(II), which may have had influence on the binding affinity and specificity of the enzyme. |
| − | As previously noted, AA10s exist both as single module entities and in multimodular forms. CBP21 from ''S. marcescens'' and EfCBM33A from ''E. faecalis'' are catalytically functional single-module AA10s that both bind well to chitin (CBP21 is specific for beta-chitin, whereas EfCBM33A binds to both alpha and beta-chitin). The cellulose targeting AA10 from ''S. coelicolor'' (CelS2) on the other hand, is bimodular and contains a cellulose binding CBM2 in addition to the catalytic AA10 module <cite>Forsberg2011</cite>. The V. cholerae AA10 is an elongated tetra-modular protein where the N-terminal AA10 module binds mucin, the two following modules bind bacterial cell walls and the C-terminal CBM5/12 bind chitin <cite>Wong2012</cite>. | + | As previously noted, AA10s exist both as single module entities and in multimodular forms. CBP21 from ''S. marcescens'' <cite>Vaaje-Kolstad2010</cite> and EfCBM33A from ''E. faecalis'' <cite>Vaaje-Kolstad2012 </cite> are catalytically functional single-module AA10s that both bind well to chitin (CBP21 is specific for beta-chitin, whereas EfCBM33A binds to both alpha and beta-chitin). The cellulose targeting AA10 from ''S. coelicolor'' (CelS2) on the other hand, is bimodular and contains a cellulose binding CBM2 in addition to the catalytic AA10 module <cite>Forsberg2011 Forsberg2014 Forsbergb2014</cite>. The V. cholerae AA10 is an elongated tetra-modular protein where the N-terminal catalytically actove AA10 module binds mucin, the two following modules bind bacterial cell walls and the C-terminal CBM5/12 bind chitin <cite>Wong2012 Loose2014</cite>. |
| − | The substrate binding surface of AA10s is flat and | + | The substrate binding surface of AA10s is flat and lacks the typical arrangement of aromatic amino acids that is common for carbohydrate binding proteins <cite>Vaaje-Kolstad2005-1 Vaaje-Kolstad2017</cite>. It is therefore thought that substrate binding is predominantly mediated by hydrogen bonds, as is substantiated by a recent study of CelS2 <cite>Forsberg2018</cite>. The flat substrate binding surface seems optimal for binding the flat surface of crystalline carbohydrate structures like cellulose and chitin (Fig. 1). |
| Line 82: | Line 82: | ||
#Forsberg2018 pmid=29222333 | #Forsberg2018 pmid=29222333 | ||
#Vaaje-Kolstad2009 pmid=19348025 | #Vaaje-Kolstad2009 pmid=19348025 | ||
| + | #Loose2014 pmid=25109775 | ||
| + | |||
| + | #Vaaje-Kolstad2017 pmid=28086105 | ||
</biblio> | </biblio> | ||
[[Category:Auxiliary Activity Families|AA010]] | [[Category:Auxiliary Activity Families|AA010]] | ||
Revision as of 12:00, 15 January 2018
This page is currently under construction. This means that the Responsible Curator has deemed that the page's content is not quite up to CAZypedia's standards for full public consumption. All information should be considered to be under revision and may be subject to major changes.
- Author: ^^^Vincent Eijsink^^^ and ^^^Gustav Vaaje-Kolstad^^^
- Responsible Curator: ^^^Vincent Eijsink^^^
| Auxiliary Activity Family 10 | |
| Clan | Structurally related to AA9 & AA11 & AA13 |
| Mechanism | lytic oxidase |
| Active site residues | mononuclear copper ion |
| CAZy DB link | |
| http://www.cazy.org/AA10.html | |
Substrate specificities
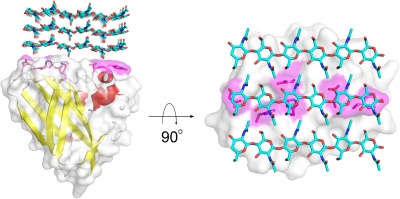
Members of the AA10 family of lytic polysaccharide monooxygenases were previously classified as Carbohydrate Binding Module Family 33 members). They are known to cleave chitin [3, 4, 5] and cellulose [6, 7, 8]. Most characterized AA10 LPMO oxidize the C1 of the scissile glycosidic bond, but some also oxidize C4 [9, 10]. AA10 LPMOs are closely related to LPMOs in families AA11 and AA13, known to cleave chitin and starch, respectively. AA10 modules often occur in combination with additional modules, in particular carbohydrate-binding modules (CBMs), but also catalytic domains such as GH18 chitinases [2]. The CBMs contribute to substrate-binding and may also affect operational stability of the LPMO [10, 11].
Before proteins belonging to AA10 were identified as enzymes, they were generally known as chitin binding proteins (CBPs). The reason for this was that most AA10s studied had been identified in chitinolytic systems such as that of Serratia marcescens [12, 13], several Streptomyces species [14, 15, 16], Bacillus amyloliquefaciens [17],Vibrio cholerae [18], Pseudomonas aeruginosa [19] and Lacotococcus lactis [20]. Upon their characterization no other function than substrate binding could be identified, thus the name "chitin binding protein" was coined. Substrates and potential substrates identified by binding studies include alpha-chitin [14, 15], beta-chitin [1, 13], both the alpha- and beta-chitin allomorphs [4, 17, 20] chitosan [16], cellulose [6, 21] and even bacterial and eptihelial cell surfaces where the binding interaction substrate has been suggested to be GlcNAc containing glycoproteins or proteoglycans [18, 22]. It should be noted that studies on AA10s prior to their identification as copper-dependent metalloenzymes were conducted in the absence of Cu(II), which may have had influence on the binding affinity and specificity of the enzyme.
As previously noted, AA10s exist both as single module entities and in multimodular forms. CBP21 from S. marcescens [3] and EfCBM33A from E. faecalis [4] are catalytically functional single-module AA10s that both bind well to chitin (CBP21 is specific for beta-chitin, whereas EfCBM33A binds to both alpha and beta-chitin). The cellulose targeting AA10 from S. coelicolor (CelS2) on the other hand, is bimodular and contains a cellulose binding CBM2 in addition to the catalytic AA10 module [6, 9, 11]. The V. cholerae AA10 is an elongated tetra-modular protein where the N-terminal catalytically actove AA10 module binds mucin, the two following modules bind bacterial cell walls and the C-terminal CBM5/12 bind chitin [18, 23].
The substrate binding surface of AA10s is flat and lacks the typical arrangement of aromatic amino acids that is common for carbohydrate binding proteins [1, 24]. It is therefore thought that substrate binding is predominantly mediated by hydrogen bonds, as is substantiated by a recent study of CelS2 [10]. The flat substrate binding surface seems optimal for binding the flat surface of crystalline carbohydrate structures like cellulose and chitin (Fig. 1).
Kinetics and Mechanism
Content is to be added here.
Catalytic Residues and copper coordination
Under construction. References [3, 5, 25, 26]
Three-dimensional structures
In 2005 the structure CBP21 from S. marcescens was solved and represents the first structure in the AA10 family 2BEM [1]. The CBP21 wild type structure has three molecules in the asymetric unit, which of only chain C show electron density for a metal bound in the metal binding motif (modeled as a sodium ion, but is probably a reduced copper ion with low occupancy). Later the same year the structure of the CBP21-Y54A mutant was solved (different crystal form and space group), showing two molecules in the asymetric unit with no trace of electron density for a metal ion bound in the active site 2BEN [27]. The second AA10 structure, one of two AA10 from Burkholderia pseudomallei 1710b (Uniprot ID: Q3JY22), was published in the PDB late in 2011 by Seattle Structural Genomics Center for Infectious Disease 3UAM. The structure contains five molecules in the asymetric unit that all have two amino acids from the signal peptide still attached to the N-terminus, most likely disrupting the active site. The third unique AA10 structure to be solved was GbpA from Vibrio cholerae O1 biovar El Tor str. N16961 2XWX [18]. GbpA is unique in the sense that it contains four discreet modules (the N-termainl AA10 module, two modules with unkown funtion and a C-terminal CBM5/12) in addition to the N-terminal AA10 module. The published structure of GbpA only lacks the C-termainal CBM5/12 and is thus the first multimodular AA10 structure to be published. Shortly after the release of the GbpA, the structure of EfCBM33A from Enterococcus faecalis 4A02 [28] was published. The structure of EfCBM33A was solved at very high resolution (0.95Å), but similar to the other structures solved, no metal ion was observed bound in the active site. In 2012 the solution structure of CBP21 wild type (apo-form) was solved by NMR 2LHS [5] and in spring 2013 the structure of the single AA10 harbored byBacillus amyloliquefaciens DSM7 (BaCBM33) was published [25]. The latter publication contained three structures; the apo-enzyme 2YOW and two structures containing a reduced copper ion bond to the active site 2YOY2YOX, thus being the first AA10 structures with the copper ion (that is essential for activity) bound in the active site.
Family Firsts
- First AA10 protein identified
- The first proteins studied from the AA10 family were all isolated and cloned from various Streptomyces strains, a major effort carried out by the Schrempf group of Osnabrück University. The first family AA10 protein to be isolated and characterized was CHB1 from Streptomyces olivaceoviridis a study published in 1994 [29]. CHB1 was shown to bind strongly to alpha-chitin and was also observed to bind to fungal hyphae.
- First demonstration of synergy between AA10 and canonical glycoside hydrolases
- In 2005, Vaaje-Kolstad and co-workers showed that CBP21 from S. marcescens was able to increase the rate of chitin hydrolysis by a variety of chitinases, including all three S. marsescens GH18 chitinases and a GH19 chitinase from Streptomyces coelicolor [27].
- First demonstration of oxidative cleavage by an AA10 protein
- Catalysis of lytic oxidation of a glycosidic bond by an AA10 enzyme was first shown for CBP21 in 2010, where oxidative cleavage of chitin was demonstrated [3] . This finding also represents the first demonstration of LPMO activity, regardless of AA family.
- First 3-D structure
- CBP21, the single AA10-type LPMO from the Gram negative bacterium Serratia marcescens, PDB ID 2BEM.
References
- Vaaje-Kolstad G, Houston DR, Riemen AH, Eijsink VG, and van Aalten DM. (2005). Crystal structure and binding properties of the Serratia marcescens chitin-binding protein CBP21. J Biol Chem. 2005;280(12):11313-9. DOI:10.1074/jbc.M407175200 |
- Horn SJ, Vaaje-Kolstad G, Westereng B, and Eijsink VG. (2012). Novel enzymes for the degradation of cellulose. Biotechnol Biofuels. 2012;5(1):45. DOI:10.1186/1754-6834-5-45 |
- Vaaje-Kolstad G, Westereng B, Horn SJ, Liu Z, Zhai H, Sørlie M, and Eijsink VG. (2010). An oxidative enzyme boosting the enzymatic conversion of recalcitrant polysaccharides. Science. 2010;330(6001):219-22. DOI:10.1126/science.1192231 |
- Vaaje-Kolstad G, Bøhle LA, Gåseidnes S, Dalhus B, Bjørås M, Mathiesen G, and Eijsink VG. (2012). Characterization of the chitinolytic machinery of Enterococcus faecalis V583 and high-resolution structure of its oxidative CBM33 enzyme. J Mol Biol. 2012;416(2):239-54. DOI:10.1016/j.jmb.2011.12.033 |
- Aachmann FL, Sørlie M, Skjåk-Bræk G, Eijsink VG, and Vaaje-Kolstad G. (2012). NMR structure of a lytic polysaccharide monooxygenase provides insight into copper binding, protein dynamics, and substrate interactions. Proc Natl Acad Sci U S A. 2012;109(46):18779-84. DOI:10.1073/pnas.1208822109 |
- Forsberg Z, Vaaje-Kolstad G, Westereng B, Bunæs AC, Stenstrøm Y, MacKenzie A, Sørlie M, Horn SJ, and Eijsink VG. (2011). Cleavage of cellulose by a CBM33 protein. Protein Sci. 2011;20(9):1479-83. DOI:10.1002/pro.689 |
- Forsberg Z, Mackenzie AK, Sørlie M, Røhr ÅK, Helland R, Arvai AS, Vaaje-Kolstad G, and Eijsink VG. (2014). Structural and functional characterization of a conserved pair of bacterial cellulose-oxidizing lytic polysaccharide monooxygenases. Proc Natl Acad Sci U S A. 2014;111(23):8446-51. DOI:10.1073/pnas.1402771111 |
- Forsberg Z, Bissaro B, Gullesen J, Dalhus B, Vaaje-Kolstad G, and Eijsink VGH. (2018). Structural determinants of bacterial lytic polysaccharide monooxygenase functionality. J Biol Chem. 2018;293(4):1397-1412. DOI:10.1074/jbc.M117.817130 |
- Forsberg Z, Røhr AK, Mekasha S, Andersson KK, Eijsink VG, Vaaje-Kolstad G, and Sørlie M. (2014). Comparative study of two chitin-active and two cellulose-active AA10-type lytic polysaccharide monooxygenases. Biochemistry. 2014;53(10):1647-56. DOI:10.1021/bi5000433 |
- Fuchs RL, McPherson SA, and Drahos DJ. (1986). Cloning of a Serratia marcescens Gene Encoding Chitinase. Appl Environ Microbiol. 1986;51(3):504-9. DOI:10.1128/aem.51.3.504-509.1986 |
- Suzuki K, Suzuki M, Taiyoji M, Nikaidou N, and Watanabe T. (1998). Chitin binding protein (CBP21) in the culture supernatant of Serratia marcescens 2170. Biosci Biotechnol Biochem. 1998;62(1):128-35. DOI:10.1271/bbb.62.128 |
- Zeltins A and Schrempf H. (1997). Specific interaction of the Streptomyces chitin-binding protein CHB1 with alpha-chitin--the role of individual tryptophan residues. Eur J Biochem. 1997;246(2):557-64. DOI:10.1111/j.1432-1033.1997.t01-1-00557.x |
- Kolbe S, Fischer S, Becirevic A, Hinz P, and Schrempf H. (1998). The Streptomyces reticuli alpha-chitin-binding protein CHB2 and its gene. Microbiology (Reading). 1998;144 ( Pt 5):1291-1297. DOI:10.1099/00221287-144-5-1291 |
- Saito A, Miyashita K, Biukovic G, and Schrempf H. (2001). Characteristics of a Streptomyces coelicolor A3(2) extracellular protein targeting chitin and chitosan. Appl Environ Microbiol. 2001;67(3):1268-73. DOI:10.1128/AEM.67.3.1268-1273.2001 |
- Chu HH, Hoang V, Hofemeister J, and Schrempf H. (2001). A Bacillus amyloliquefaciens ChbB protein binds beta- and alpha-chitin and has homologues in related strains. Microbiology (Reading). 2001;147(Pt 7):1793-1803. DOI:10.1099/00221287-147-7-1793 |
- Wong E, Vaaje-Kolstad G, Ghosh A, Hurtado-Guerrero R, Konarev PV, Ibrahim AF, Svergun DI, Eijsink VG, Chatterjee NS, and van Aalten DM. (2012). The Vibrio cholerae colonization factor GbpA possesses a modular structure that governs binding to different host surfaces. PLoS Pathog. 2012;8(1):e1002373. DOI:10.1371/journal.ppat.1002373 |
- Folders J, Tommassen J, van Loon LC, and Bitter W. (2000). Identification of a chitin-binding protein secreted by Pseudomonas aeruginosa. J Bacteriol. 2000;182(5):1257-63. DOI:10.1128/JB.182.5.1257-1263.2000 |
- Vaaje-Kolstad G, Bunaes AC, Mathiesen G, and Eijsink VG. (2009). The chitinolytic system of Lactococcus lactis ssp. lactis comprises a nonprocessive chitinase and a chitin-binding protein that promotes the degradation of alpha- and beta-chitin. FEBS J. 2009;276(8):2402-15. DOI:10.1111/j.1742-4658.2009.06972.x |
- Walter S and Schrempf H. (2008). Characteristics of the surface-located carbohydrate-binding protein CbpC from Streptomyces coelicolor A32. Arch Microbiol. 2008;190(2):119-27. DOI:10.1007/s00203-008-0373-7 |
- Sánchez B, González-Tejedo C, Ruas-Madiedo P, Urdaci MC, and Margolles A. (2011). Lactobacillus plantarum extracellular chitin-binding protein and its role in the interaction between chitin, Caco-2 cells, and mucin. Appl Environ Microbiol. 2011;77(3):1123-6. DOI:10.1128/AEM.02080-10 |
- Loose JS, Forsberg Z, Fraaije MW, Eijsink VG, and Vaaje-Kolstad G. (2014). A rapid quantitative activity assay shows that the Vibrio cholerae colonization factor GbpA is an active lytic polysaccharide monooxygenase. FEBS Lett. 2014;588(18):3435-40. DOI:10.1016/j.febslet.2014.07.036 |
- Vaaje-Kolstad G, Forsberg Z, Loose JS, Bissaro B, and Eijsink VG. (2017). Structural diversity of lytic polysaccharide monooxygenases. Curr Opin Struct Biol. 2017;44:67-76. DOI:10.1016/j.sbi.2016.12.012 |
- Hemsworth GR, Taylor EJ, Kim RQ, Gregory RC, Lewis SJ, Turkenburg JP, Parkin A, Davies GJ, and Walton PH. (2013). The copper active site of CBM33 polysaccharide oxygenases. J Am Chem Soc. 2013;135(16):6069-77. DOI:10.1021/ja402106e |
- Hemsworth GR, Davies GJ, and Walton PH. (2013). Recent insights into copper-containing lytic polysaccharide mono-oxygenases. Curr Opin Struct Biol. 2013;23(5):660-8. DOI:10.1016/j.sbi.2013.05.006 |
- Vaaje-Kolstad G, Horn SJ, van Aalten DM, Synstad B, and Eijsink VG. (2005). The non-catalytic chitin-binding protein CBP21 from Serratia marcescens is essential for chitin degradation. J Biol Chem. 2005;280(31):28492-7. DOI:10.1074/jbc.M504468200 |
- Schnellmann J, Zeltins A, Blaak H, and Schrempf H. (1994). The novel lectin-like protein CHB1 is encoded by a chitin-inducible Streptomyces olivaceoviridis gene and binds specifically to crystalline alpha-chitin of fungi and other organisms. Mol Microbiol. 1994;13(5):807-19. DOI:10.1111/j.1365-2958.1994.tb00473.x |