CAZypedia needs your help! We have many unassigned GH, PL, CE, AA, GT, and CBM pages in need of Authors and Responsible Curators.
Scientists at all career stages, including students, are welcome to contribute to CAZypedia. Read more here, and in the 10th anniversary article in Glycobiology.
New to the CAZy classification? Read this first.
*
Consider attending the 15th Carbohydrate Bioengineering Meeting in Ghent, 5-8 May 2024.
Difference between revisions of "Carbohydrate Binding Module Family 15"
| Line 21: | Line 21: | ||
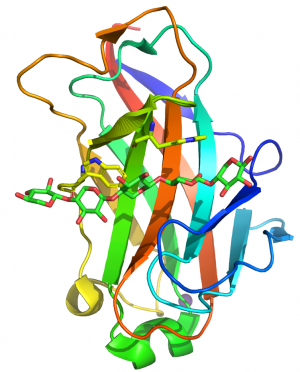
== Structural Features == | == Structural Features == | ||
| − | [[File:CBM15structure.png|thumb|300px|right|'''Figure 1.''' The fold of the CBM15 in C. | + | [[File:CBM15structure.png|thumb|300px|right|'''Figure 1.''' The fold of the CBM15 in ''C. japonicus'' Xyn10C (PDB code 1GNY) highlighting the two perpendicular tryptophans bound to xylose residues n and n+2 in the 3-fold helical structure of xylan.]] |
CBM15s comprise ~150 amino acids. The crystal structure of the protein module from the ''C. japonicus'' GH10 xylanase Xyn10C was determined in complex with xylohexaose <cite>Szabo2001</cite>. The structure of CBM15 forms a classic β-jelly roll, predominantly consisting of five major anti-parallel β-strands on the two faces CBM15 contains a deep cleft that runs along the concave face of the β-sheet, 20–25 Å long, which forms the binding site for the target ligands. The protein was crystallized in the presence of xylohexaose, and the structure reveals five well-ordered xylose rings (defined as Xyl1 to Xyl5 from the reducing to non-reducing end). Two solvent-exposed tryptophan residues, Trp176 and Trp181, lie in the binding groove and make hydrophobic stacking interactions with Xyl2 and Xyl4, respectively. The indole rings of the two tryptophans were perpendicular to each other and their position is consistent with binding n and n+2 xylose residues in the 3-fold helix structure of xylan (Figure 1). The conformation of these aromatic residues are very similar to the pair of tryptophans that interact with xylan in the CBM2s in ''Cellulomonas fimi'' Xyn11A <cite>Simpson1999</cite>. Only Xyl 2 and Xyl3 of the bound xylopentaose made direct interactions with the CBM. It was argued that the paucity of polar interactions enabled the CBM to bind to highly decorated xylans. Mutagenesis studies showed that the two surface trypotphans were essential for xylan recognition. Mutation of the polar residues that had a direct or indirect interaction with xylans or xylopentaose reduced affinity by 100- and 10-fold, respectively <cite>Pell2003</cite>. | CBM15s comprise ~150 amino acids. The crystal structure of the protein module from the ''C. japonicus'' GH10 xylanase Xyn10C was determined in complex with xylohexaose <cite>Szabo2001</cite>. The structure of CBM15 forms a classic β-jelly roll, predominantly consisting of five major anti-parallel β-strands on the two faces CBM15 contains a deep cleft that runs along the concave face of the β-sheet, 20–25 Å long, which forms the binding site for the target ligands. The protein was crystallized in the presence of xylohexaose, and the structure reveals five well-ordered xylose rings (defined as Xyl1 to Xyl5 from the reducing to non-reducing end). Two solvent-exposed tryptophan residues, Trp176 and Trp181, lie in the binding groove and make hydrophobic stacking interactions with Xyl2 and Xyl4, respectively. The indole rings of the two tryptophans were perpendicular to each other and their position is consistent with binding n and n+2 xylose residues in the 3-fold helix structure of xylan (Figure 1). The conformation of these aromatic residues are very similar to the pair of tryptophans that interact with xylan in the CBM2s in ''Cellulomonas fimi'' Xyn11A <cite>Simpson1999</cite>. Only Xyl 2 and Xyl3 of the bound xylopentaose made direct interactions with the CBM. It was argued that the paucity of polar interactions enabled the CBM to bind to highly decorated xylans. Mutagenesis studies showed that the two surface trypotphans were essential for xylan recognition. Mutation of the polar residues that had a direct or indirect interaction with xylans or xylopentaose reduced affinity by 100- and 10-fold, respectively <cite>Pell2003</cite>. | ||
Revision as of 04:27, 17 January 2018
This page is currently under construction. This means that the Responsible Curator has deemed that the page's content is not quite up to CAZypedia's standards for full public consumption. All information should be considered to be under revision and may be subject to major changes.
- Author: ^^^Harry Gilbert^^^
- Responsible Curator: ^^^Harry Gilbert^^^
| CAZy DB link | |
| http://www.cazy.org/CBM15.html |
Ligand specificities
The three family 15 CBMs (CBM15s) are all derived from the Cellvibrio bacterial genus. The one fully characterized CBM15 from Cellvibrio japonicus Xyl10C [1] bound to different forms of xylan with a preference for oat spelt xylan (KA of ~1.4 x 104 M-1). The CBM also bound to xylooligosacchrides exhibiting affinities for xylohexaose and xylopentaose that were similar to oat spelt xylan, and significantly weaker affinity for xylotetraose and xylotriose. The protein displayed weak but measurable affinity (KA of ~2 x 103 M-1) for barley β1,3-β1,4-mixed linked glucan and cellohexaose. The CBM15 did not bind to insoluble xylan or amorphous cellulose. Isothermal titration calorimetry showed that binding to xylan and glucan ligands was driven by enthalpic forces with changes in entropy have a negative impact on affinity [1]. The stoichiometry of binding to soluble xylans was consistent with an endo binding mode, demonstrating that the family 15 module is a type B CBM.
Structural Features
CBM15s comprise ~150 amino acids. The crystal structure of the protein module from the C. japonicus GH10 xylanase Xyn10C was determined in complex with xylohexaose [1]. The structure of CBM15 forms a classic β-jelly roll, predominantly consisting of five major anti-parallel β-strands on the two faces CBM15 contains a deep cleft that runs along the concave face of the β-sheet, 20–25 Å long, which forms the binding site for the target ligands. The protein was crystallized in the presence of xylohexaose, and the structure reveals five well-ordered xylose rings (defined as Xyl1 to Xyl5 from the reducing to non-reducing end). Two solvent-exposed tryptophan residues, Trp176 and Trp181, lie in the binding groove and make hydrophobic stacking interactions with Xyl2 and Xyl4, respectively. The indole rings of the two tryptophans were perpendicular to each other and their position is consistent with binding n and n+2 xylose residues in the 3-fold helix structure of xylan (Figure 1). The conformation of these aromatic residues are very similar to the pair of tryptophans that interact with xylan in the CBM2s in Cellulomonas fimi Xyn11A [2]. Only Xyl 2 and Xyl3 of the bound xylopentaose made direct interactions with the CBM. It was argued that the paucity of polar interactions enabled the CBM to bind to highly decorated xylans. Mutagenesis studies showed that the two surface trypotphans were essential for xylan recognition. Mutation of the polar residues that had a direct or indirect interaction with xylans or xylopentaose reduced affinity by 100- and 10-fold, respectively [3].
Functionalities
The three CBM15s are all appended to GH10 xylanases [1, 4]. The CBM15 from C. japonicus Xyn10C and a xylan binding CBM2 from Cellulomonas fimi Xyn11D were shown to bind to plant cell walls at similar locations [5]. The CBM15, when fused to a xylanase, caused a very modest increase in activity [6], suggesting that enzyme potentiation was not its primary biological role. As the CBM15-containing enzyme, Xyn10C, is appended to the surface of C. japonicus, it was proposed that the protein module contributes to ensuring xylooligosaccharides generated by the xylanase are retained for transport into the bacterial periplasm [7]
Family Firsts
- First Identified
The first CBM15 was observed in the Cellvibrio mixtus GH10 xylanase XylB, and qualitative studies indicated that the protein module bound to glucans [4]. The first detailed analysis of a CBM15 was from the C. japonicus xylanase Xyn10C [1].
First Structural Characterization:
The first 3D structure of a CBM15 was determined by X-ray crystallography from the C. japonicus xylanase Xyn10A [1]. This also represents the first structure of a xylan binding CBM in complex with its ligand.
References
- Szabo L, Jamal S, Xie H, Charnock SJ, Bolam DN, Gilbert HJ, and Davies GJ. (2001). Structure of a family 15 carbohydrate-binding module in complex with xylopentaose. Evidence that xylan binds in an approximate 3-fold helical conformation. J Biol Chem. 2001;276(52):49061-5. DOI:10.1074/jbc.M109558200 |
- Simpson PJ, Bolam DN, Cooper A, Ciruela A, Hazlewood GP, Gilbert HJ, and Williamson MP. (1999). A family IIb xylan-binding domain has a similar secondary structure to a homologous family IIa cellulose-binding domain but different ligand specificity. Structure. 1999;7(7):853-64. DOI:10.1016/s0969-2126(99)80108-7 |
- Pell G, Williamson MP, Walters C, Du H, Gilbert HJ, and Bolam DN. (2003). Importance of hydrophobic and polar residues in ligand binding in the family 15 carbohydrate-binding module from Cellvibrio japonicus Xyn10C. Biochemistry. 2003;42(31):9316-23. DOI:10.1021/bi0347510 |
- Millward-Sadler SJ, Davidson K, Hazlewood GP, Black GW, Gilbert HJ, and Clarke JH. (1995). Novel cellulose-binding domains, NodB homologues and conserved modular architecture in xylanases from the aerobic soil bacteria Pseudomonas fluorescens subsp. cellulosa and Cellvibrio mixtus. Biochem J. 1995;312 ( Pt 1)(Pt 1):39-48. DOI:10.1042/bj3120039 |
- McCartney L, Blake AW, Flint J, Bolam DN, Boraston AB, Gilbert HJ, and Knox JP. (2006). Differential recognition of plant cell walls by microbial xylan-specific carbohydrate-binding modules. Proc Natl Acad Sci U S A. 2006;103(12):4765-70. DOI:10.1073/pnas.0508887103 |
- Hervé C, Rogowski A, Blake AW, Marcus SE, Gilbert HJ, and Knox JP. (2010). Carbohydrate-binding modules promote the enzymatic deconstruction of intact plant cell walls by targeting and proximity effects. Proc Natl Acad Sci U S A. 2010;107(34):15293-8. DOI:10.1073/pnas.1005732107 |
- Pell G, Szabo L, Charnock SJ, Xie H, Gloster TM, Davies GJ, and Gilbert HJ. (2004). Structural and biochemical analysis of Cellvibrio japonicus xylanase 10C: how variation in substrate-binding cleft influences the catalytic profile of family GH-10 xylanases. J Biol Chem. 2004;279(12):11777-88. DOI:10.1074/jbc.M311947200 |