CAZypedia needs your help! We have many unassigned GH, PL, CE, AA, GT, and CBM pages in need of Authors and Responsible Curators.
Scientists at all career stages, including students, are welcome to contribute to CAZypedia. Read more here, and in the 10th anniversary article in Glycobiology.
New to the CAZy classification? Read this first.
*
Consider attending the 15th Carbohydrate Bioengineering Meeting in Ghent, 5-8 May 2024.
Difference between revisions of "Carbohydrate Binding Module Family 5"
Manjeet Kaur (talk | contribs) |
Harry Brumer (talk | contribs) m (Text replacement - "\^\^\^(.*)\^\^\^" to "$1") |
||
| (50 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
<!-- RESPONSIBLE CURATORS: Please replace the {{UnderConstruction}} tag below with {{CuratorApproved}} when the page is ready for wider public consumption --> | <!-- RESPONSIBLE CURATORS: Please replace the {{UnderConstruction}} tag below with {{CuratorApproved}} when the page is ready for wider public consumption --> | ||
| − | {{ | + | {{CuratorApproved}} |
| − | * [[Author]]: | + | * [[Author]]: [[User:Manjeet Kaur|Manjeet Kaur]] |
| − | * [[Responsible Curator]]: | + | * [[Responsible Curator]]: [[User:Appa Rao Podile|Appa Rao Podile]] |
---- | ---- | ||
| Line 18: | Line 18: | ||
== Ligand specificities == | == Ligand specificities == | ||
| − | The family 5 carbohydrate binding modules (CBM5) | + | The family 5 carbohydrate binding modules (CBM5) have approximately 60 amino acid residues. The CBM5 are found as accessory domains in chitinases <cite>Kezuka2006 Van2000 Uni2012 Manjeet2013</cite>, endoglucanases <cite>Brun1997</cite> and lytic polysaccharide mono-oxygenases (LPMOs) <cite>Forsberg2016 Mutahir2018 Manjeet2019</cite>. This family first originated as a cellulose binding domain family V (CBD V) <cite>Kezuka2006 Simpson1999</cite>. However, CBM5 members have now been reported to have affinity to both cellulose and chitin <cite>Kezuka2006 Van2000 Uni2012 Manjeet2013 Brun1997 Forsberg2016 Mutahir2018 Manjeet2019 Simpson1999</cite>. Deletion of CBM5 from chitinases or LPMOs has shown considerable reduction in chitin binding <cite>Manjeet2013 Forsberg2016 Manjeet2019</cite>. The ''K<sub>d</sub>'' and ''B<sub>max</sub>'' values for CBM5 of an LPMO from ''Cellvibrio japonicas'', ''Cj''LPMO10A for α-chitin, were 5.3 µM and 4.8 µmol/g α-chitin, respectively <cite>Forsberg2016</cite>. The ''K<sub>d</sub>'' values of CBM5 from ''Bacillus thuringiensis'', ''Bt''CBM5 for α- and β-chitin were in the order of 0.6-0.7 µM, whereas the ''B<sub>max</sub>'' value was ~1.9 µmol/g for both α- and β-chitin <cite>Manjeet2019</cite>. |
== Structural Features == | == Structural Features == | ||
| − | The structures of CBM5 domains have been elucidated for an endoglucanase, CBDEGZ from ''Erwinia chrysanthemi'' (''Ec''EGZCBM5) and two chitinases ChBDChiB from ''Serratia marcescens'' (''Sm''ChiBCBM5) and ChBDChiC from ''Streptomyces griseus'' HUT6037 (''Sg''ChiCCBM5) <cite>Kezuka2006 Van2000 Brun1997</cite>. | + | [[File:Figure 1 EcEGZCBM5.png|thumb|300px|right|'''Figure 1.''' CBM5 of an endoglucanase from ''Erwinia chrysanthemi'' [{{PDBlink}}1AIW PDB ID 1AIW] showing surface exposed aromatic residues (Trp18, Trp43 and Tyr44) and a conserved disulfide bond between Cys4 and Cys61.]] |
| + | The structures of CBM5 domains have been elucidated for an endoglucanase, CBDEGZ from ''Erwinia chrysanthemi'' (''Ec''EGZCBM5) and two chitinases, ChBDChiB from ''Serratia marcescens'' (''Sm''ChiBCBM5) and ChBDChiC from ''Streptomyces griseus'' HUT6037 (''Sg''ChiCCBM5) <cite>Kezuka2006 Van2000 Brun1997</cite>. | ||
| − | The three structures revealed that | + | The three structures revealed that CBM5s are composed of five β-strands (β1-5) <cite>Kezuka2006 Van2000 Brun1997</cite>. The β1, β2 and β3 forms the principle structure and the additional short β-strands (β4 and β5) form an antiparallel β-sheet which is independent of the main strand. The ''Ec''EGZCBM5 resembles a ski-boot or L-shaped structure composed of only β-sheets <cite>Brun1997</cite>. Helix structures have not been found in CBM5 modules. |
| − | There are a few differences in CBM5 | + | There are a few differences in CBM5 structures from endo-glucanases (''Ec''EGZCBM5) and chitinases (''Sm''ChiBCBM5 and ''Sg''ChiCCBM5). The ''Ec''EGZCBM5 possesses a conserved disulfide bond between Cys4 and Cys61 (Figure 1) <cite>Brun1997</cite>. These disulfide bonds have not been reported in ''Sm''ChiBCBM5 and ''Sg''ChiCCBM5 <cite>Kezuka2006 Van2000</cite>. |
| − | CBM5 modules possess surface exposed aromatic residues which interact with polysaccharides most probably through hydrophobic interactions. The ''Ec''EGZCBM5 possesses three exposed aromatic residues Trp18, Trp43 and Tyr44. Trp18 is present on an extra loop | + | CBM5 modules possess surface exposed aromatic residues which interact with polysaccharides most probably through hydrophobic interactions <cite>Kezuka2006 Simpson1999</cite>. These aromatic residues form a flat platform to bind to the planar surfaces of crystalline cellulose/chitin. CBM5 are thus classified under [[Carbohydrate-binding_modules#Types|type A]] CBMs <cite>Gilbert2013</cite>. The ''Ec''EGZCBM5 [{{PDBlink}}1AIW PDB ID 1AIW] possesses three exposed aromatic residues: Trp18, Trp43 and Tyr44 (Figure 1). Trp18 is present on an extra loop, is linearly aligned to Trp43 and Tyr44 and extends the substrate binding site <cite>Brun1997</cite>. The three residues are essential for complete binding of ''Ec''EGZCBM5. Polar residues like Asp17 are present on the cellulose binding face and form H-bonds to stabilize the appropriate orientation of cellulose binding residues <cite>Brun1997</cite>. Polar residues also form H-bonds with oxygen atoms and/or OH-groups of glucose subunits of cellulose and thus have been proposed to play a role in cellulose-disruption <cite>Brun1997</cite>. Mutation of Asp17 resulted in decreased binding towards cellulose <cite>Simpson1999</cite>. |
| − | ''Sm''ChiBCBM5 and ''Sg''ChiCCBM5 have only two surface exposed aromatic residues, Trp479 and Trp481 in ''Sm''ChiBCBM5 and Trp59 and Trp60 in ''Sg''ChiCCBM5; whose structural homologues in ''Ec''EGZCBM5 are Trp43 and Tyr44 <cite>Van2000</cite>. The two exposed aromatic residues are sufficient for binding in ''Sm''ChiBCBM5 and ''Sg''ChiCCBM5. These residues interact extensively and play a vital role in increasing the proximity of substrate through hydrophobic interactions. It has been proposed that either of the two exposed residues should be a tryptophan residue | + | ''Sm''ChiBCBM5 and ''Sg''ChiCCBM5 have only two surface exposed aromatic residues, Trp479 and Trp481 in ''Sm''ChiBCBM5 and Trp59 and Trp60 in ''Sg''ChiCCBM5; whose structural homologues in ''Ec''EGZCBM5 are Trp43 and Tyr44 <cite>Van2000</cite>. The two exposed aromatic residues are sufficient for binding in ''Sm''ChiBCBM5 and ''Sg''ChiCCBM5 <cite>Kezuka2006</cite>. These residues interact extensively and play a vital role in increasing the proximity of substrate through hydrophobic interactions. It has been proposed that either of the two exposed residues should be a tryptophan residue as the Tyr-Tyr pair has not been found in the family <cite>Kezuka2006</cite>. |
| − | In ''Sg''ChiCCBM5, six residues (Trp36, Val 48, Tyr 50, Tyr55, Pro66 and Trp72) participate in forming a hydrophobic core in the domain centre. The side chain of Pro66 is internally buried while the remaining 5 residues form the hydrophobic socket <cite>Kezuka2006</cite>. Only two surface exposed aromatic residues (Trp59 and Trp60) | + | In ''Sg''ChiCCBM5, six residues (Trp36, Val 48, Tyr 50, Tyr55, Pro66 and Trp72) participate in forming a hydrophobic core in the domain centre. The side chain of Pro66 is internally buried while the remaining 5 residues form the hydrophobic socket <cite>Kezuka2006</cite>. Only two surface exposed aromatic residues (Trp59 and Trp60), which are positioned on a loop between the sheets β2 and β5, are involved in carbohydrate binding <cite>Kezuka2006</cite>. When protein-substrate interactions were studied between ''Sg''ChiCCBM5 and tri-N-acetyl-chitotriose, it was found that the ligand binding was facilitated by two stacking interactions (Trp59-NAG-1 and Trp-NAG3) and two H-bonds (Trp60-N and NAG2-O7 and Trp56-NE1 and NAG2-O6) <cite>Kezuka2006</cite>. |
== Functionalities == | == Functionalities == | ||
| − | Multi-modular enzymes like endo-glucanases, chitinases and LPMOs possess CBM5 modules as accessory domains appended to their catalytic domain, either directly or with the help of linkers like FnIII domains <cite>Kezuka2006 Van2000 Uni2012 Manjeet2013 Brun1997 Forsberg2016 Mutahir2018 Manjeet2019</cite>. The CBM5 domains are responsible for increased affinity of these enzymes towards crystalline cellulose or chitin. Their presence also increases the efficiency of enzymes to bind to substrates in a broader pH range <cite>Uni2012 Manjeet2019</cite>. Deletion of CBM5 | + | Multi-modular enzymes like endo-glucanases, chitinases and LPMOs possess CBM5 modules as accessory domains appended to their catalytic domain, either directly or with the help of linkers like FnIII domains <cite>Kezuka2006 Van2000 Uni2012 Manjeet2013 Brun1997 Forsberg2016 Mutahir2018 Manjeet2019</cite>. The CBM5 domains are responsible for increased affinity of these enzymes towards crystalline cellulose or chitin. Their presence also increases the efficiency of enzymes to bind to substrates in a broader pH range <cite>Uni2012 Manjeet2019</cite>. Deletion of the CBM5 resulted in reduction or complete loss of binding in several instances <cite>Manjeet2013</cite>. Deletion of C-terminal FnIII and CBM5 domains from ''Bli''Chi resulted in 5-fold reduction of hydrolytic activity on β-chitin and the mutant was unable to degrade α-chitin <cite>Manjeet2013</cite>. Accessory domains have thus been suggested to play an important role in hydrolysis by moving the enzymes in close proximity of substrates <cite>Manjeet2019</cite>. Presence of CBM5 domains in LPMOs have been shown to alter the product profile while acting on crystalline β-chitin substrates <cite>Manjeet2019</cite>. In ''Bc''LPMO10A, CBM5 promoted substrate binding as well as protected the enzyme from inactivation <cite>Mutahir2018</cite>. |
== Family Firsts == | == Family Firsts == | ||
| − | ;First indentified: CBM5 modules were first discovered in Endoglucanase, CBDEGZ from ''Erwinia chrysanthemi'' <cite>Brun1997</cite>. | + | ;First indentified: CBM5 modules were first discovered in Endoglucanase, CBDEGZ from ''Erwinia chrysanthemi'' (''Ec''EGZCBM5) <cite>Brun1997</cite>. |
| − | ;First structural characterization: The first NMR derived structure of CBM5 was from | + | ;First structural characterization: The first NMR derived structure of CBM5 was from ''Ec''EGZCBM5 [{{PDBlink}}1AIW PDB ID 1AIW] <cite>Brun1997</cite> and first crystal structure was studied for ChBDChiB from ''Serratia marcescens'' (''Sm''ChiBCBM5) [{{PDBlink}}1E15 PDB ID 1E15] <cite>Van2000</cite>. |
== References == | == References == | ||
<biblio> | <biblio> | ||
#Kezuka2006 pmid=16516924 | #Kezuka2006 pmid=16516924 | ||
| − | # Van2000 | + | #Van2000 pmid=10823940 |
| − | + | #Uni2012 pmid=22451396 | |
| − | #Uni2012 | + | #Manjeet2013 pmid=23480960 |
| − | + | #Brun1997 pmid=9405041 | |
| − | #Manjeet2013 | + | #Forsberg2016 pmid=26858252 |
| − | + | #Mutahir2018 pmid=29993123 | |
| − | #Brun1997 | + | #Manjeet2019 pmid=30708015 |
| − | + | #Simpson1999 pmid=10419961 | |
| − | #Forsberg2016 | + | #Gilbert2013 pmid=23769966 |
| − | |||
| − | #Mutahir2018 | ||
| − | |||
| − | #Manjeet2019 | ||
| − | |||
| − | #Simpson1999 | ||
| − | |||
</biblio> | </biblio> | ||
| + | |||
| + | [[Category:Carbohydrate Binding Module Families|CBM005]] | ||
Latest revision as of 14:19, 18 December 2021
This page has been approved by the Responsible Curator as essentially complete. CAZypedia is a living document, so further improvement of this page is still possible. If you would like to suggest an addition or correction, please contact the page's Responsible Curator directly by e-mail.
| CAZy DB link | |
| http://www.cazy.org/CBM05.html |
Ligand specificities
The family 5 carbohydrate binding modules (CBM5) have approximately 60 amino acid residues. The CBM5 are found as accessory domains in chitinases [1, 2, 3, 4], endoglucanases [5] and lytic polysaccharide mono-oxygenases (LPMOs) [6, 7, 8]. This family first originated as a cellulose binding domain family V (CBD V) [1, 9]. However, CBM5 members have now been reported to have affinity to both cellulose and chitin [1, 2, 3, 4, 5, 6, 7, 8, 9]. Deletion of CBM5 from chitinases or LPMOs has shown considerable reduction in chitin binding [4, 6, 8]. The Kd and Bmax values for CBM5 of an LPMO from Cellvibrio japonicas, CjLPMO10A for α-chitin, were 5.3 µM and 4.8 µmol/g α-chitin, respectively [6]. The Kd values of CBM5 from Bacillus thuringiensis, BtCBM5 for α- and β-chitin were in the order of 0.6-0.7 µM, whereas the Bmax value was ~1.9 µmol/g for both α- and β-chitin [8].
Structural Features
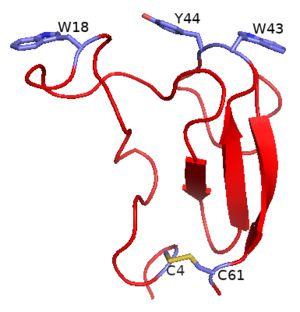
The structures of CBM5 domains have been elucidated for an endoglucanase, CBDEGZ from Erwinia chrysanthemi (EcEGZCBM5) and two chitinases, ChBDChiB from Serratia marcescens (SmChiBCBM5) and ChBDChiC from Streptomyces griseus HUT6037 (SgChiCCBM5) [1, 2, 5].
The three structures revealed that CBM5s are composed of five β-strands (β1-5) [1, 2, 5]. The β1, β2 and β3 forms the principle structure and the additional short β-strands (β4 and β5) form an antiparallel β-sheet which is independent of the main strand. The EcEGZCBM5 resembles a ski-boot or L-shaped structure composed of only β-sheets [5]. Helix structures have not been found in CBM5 modules.
There are a few differences in CBM5 structures from endo-glucanases (EcEGZCBM5) and chitinases (SmChiBCBM5 and SgChiCCBM5). The EcEGZCBM5 possesses a conserved disulfide bond between Cys4 and Cys61 (Figure 1) [5]. These disulfide bonds have not been reported in SmChiBCBM5 and SgChiCCBM5 [1, 2].
CBM5 modules possess surface exposed aromatic residues which interact with polysaccharides most probably through hydrophobic interactions [1, 9]. These aromatic residues form a flat platform to bind to the planar surfaces of crystalline cellulose/chitin. CBM5 are thus classified under type A CBMs [10]. The EcEGZCBM5 PDB ID 1AIW possesses three exposed aromatic residues: Trp18, Trp43 and Tyr44 (Figure 1). Trp18 is present on an extra loop, is linearly aligned to Trp43 and Tyr44 and extends the substrate binding site [5]. The three residues are essential for complete binding of EcEGZCBM5. Polar residues like Asp17 are present on the cellulose binding face and form H-bonds to stabilize the appropriate orientation of cellulose binding residues [5]. Polar residues also form H-bonds with oxygen atoms and/or OH-groups of glucose subunits of cellulose and thus have been proposed to play a role in cellulose-disruption [5]. Mutation of Asp17 resulted in decreased binding towards cellulose [9].
SmChiBCBM5 and SgChiCCBM5 have only two surface exposed aromatic residues, Trp479 and Trp481 in SmChiBCBM5 and Trp59 and Trp60 in SgChiCCBM5; whose structural homologues in EcEGZCBM5 are Trp43 and Tyr44 [2]. The two exposed aromatic residues are sufficient for binding in SmChiBCBM5 and SgChiCCBM5 [1]. These residues interact extensively and play a vital role in increasing the proximity of substrate through hydrophobic interactions. It has been proposed that either of the two exposed residues should be a tryptophan residue as the Tyr-Tyr pair has not been found in the family [1].
In SgChiCCBM5, six residues (Trp36, Val 48, Tyr 50, Tyr55, Pro66 and Trp72) participate in forming a hydrophobic core in the domain centre. The side chain of Pro66 is internally buried while the remaining 5 residues form the hydrophobic socket [1]. Only two surface exposed aromatic residues (Trp59 and Trp60), which are positioned on a loop between the sheets β2 and β5, are involved in carbohydrate binding [1]. When protein-substrate interactions were studied between SgChiCCBM5 and tri-N-acetyl-chitotriose, it was found that the ligand binding was facilitated by two stacking interactions (Trp59-NAG-1 and Trp-NAG3) and two H-bonds (Trp60-N and NAG2-O7 and Trp56-NE1 and NAG2-O6) [1].
Functionalities
Multi-modular enzymes like endo-glucanases, chitinases and LPMOs possess CBM5 modules as accessory domains appended to their catalytic domain, either directly or with the help of linkers like FnIII domains [1, 2, 3, 4, 5, 6, 7, 8]. The CBM5 domains are responsible for increased affinity of these enzymes towards crystalline cellulose or chitin. Their presence also increases the efficiency of enzymes to bind to substrates in a broader pH range [3, 8]. Deletion of the CBM5 resulted in reduction or complete loss of binding in several instances [4]. Deletion of C-terminal FnIII and CBM5 domains from BliChi resulted in 5-fold reduction of hydrolytic activity on β-chitin and the mutant was unable to degrade α-chitin [4]. Accessory domains have thus been suggested to play an important role in hydrolysis by moving the enzymes in close proximity of substrates [8]. Presence of CBM5 domains in LPMOs have been shown to alter the product profile while acting on crystalline β-chitin substrates [8]. In BcLPMO10A, CBM5 promoted substrate binding as well as protected the enzyme from inactivation [7].
Family Firsts
- First indentified
- CBM5 modules were first discovered in Endoglucanase, CBDEGZ from Erwinia chrysanthemi (EcEGZCBM5) [5].
- First structural characterization
- The first NMR derived structure of CBM5 was from EcEGZCBM5 PDB ID 1AIW [5] and first crystal structure was studied for ChBDChiB from Serratia marcescens (SmChiBCBM5) PDB ID 1E15 [2].
References
- Kezuka Y, Ohishi M, Itoh Y, Watanabe J, Mitsutomi M, Watanabe T, and Nonaka T. (2006). Structural studies of a two-domain chitinase from Streptomyces griseus HUT6037. J Mol Biol. 2006;358(2):472-84. DOI:10.1016/j.jmb.2006.02.013 |
- van Aalten DM, Synstad B, Brurberg MB, Hough E, Riise BW, Eijsink VG, and Wierenga RK. (2000). Structure of a two-domain chitotriosidase from Serratia marcescens at 1.9-A resolution. Proc Natl Acad Sci U S A. 2000;97(11):5842-7. DOI:10.1073/pnas.97.11.5842 |
- Uni F, Lee S, Yatsunami R, Fukui T, and Nakamura S. (2012). Mutational analysis of a CBM family 5 chitin-binding domain of an alkaline chitinase from Bacillus sp. J813. Biosci Biotechnol Biochem. 2012;76(3):530-5. DOI:10.1271/bbb.110835 |
- Manjeet K, Purushotham P, Neeraja C, and Podile AR. (2013). Bacterial chitin binding proteins show differential substrate binding and synergy with chitinases. Microbiol Res. 2013;168(7):461-8. DOI:10.1016/j.micres.2013.01.006 |
- Brun E, Moriaud F, Gans P, Blackledge MJ, Barras F, and Marion D. (1997). Solution structure of the cellulose-binding domain of the endoglucanase Z secreted by Erwinia chrysanthemi. Biochemistry. 1997;36(51):16074-86. DOI:10.1021/bi9718494 |
- Forsberg Z, Nelson CE, Dalhus B, Mekasha S, Loose JS, Crouch LI, Røhr ÅK, Gardner JG, Eijsink VG, and Vaaje-Kolstad G. (2016). Structural and Functional Analysis of a Lytic Polysaccharide Monooxygenase Important for Efficient Utilization of Chitin in Cellvibrio japonicus. J Biol Chem. 2016;291(14):7300-12. DOI:10.1074/jbc.M115.700161 |
- Mutahir Z, Mekasha S, Loose JSM, Abbas F, Vaaje-Kolstad G, Eijsink VGH, and Forsberg Z. (2018). Characterization and synergistic action of a tetra-modular lytic polysaccharide monooxygenase from Bacillus cereus. FEBS Lett. 2018;592(15):2562-2571. DOI:10.1002/1873-3468.13189 |
- Manjeet K, Madhuprakash J, Mormann M, Moerschbacher BM, and Podile AR. (2019). A carbohydrate binding module-5 is essential for oxidative cleavage of chitin by a multi-modular lytic polysaccharide monooxygenase from Bacillus thuringiensis serovar kurstaki. Int J Biol Macromol. 2019;127:649-656. DOI:10.1016/j.ijbiomac.2019.01.183 |
- Simpson HD and Barras F. (1999). Functional analysis of the carbohydrate-binding domains of Erwinia chrysanthemi Cel5 (Endoglucanase Z) and an Escherichia coli putative chitinase. J Bacteriol. 1999;181(15):4611-6. DOI:10.1128/JB.181.15.4611-4616.1999 |
- Gilbert HJ, Knox JP, and Boraston AB. (2013). Advances in understanding the molecular basis of plant cell wall polysaccharide recognition by carbohydrate-binding modules. Curr Opin Struct Biol. 2013;23(5):669-77. DOI:10.1016/j.sbi.2013.05.005 |