CAZypedia needs your help! We have many unassigned GH, PL, CE, AA, GT, and CBM pages in need of Authors and Responsible Curators.
Scientists at all career stages, including students, are welcome to contribute to CAZypedia. Read more here, and in the 10th anniversary article in Glycobiology.
New to the CAZy classification? Read this first.
*
Consider attending the 15th Carbohydrate Bioengineering Meeting in Ghent, 5-8 May 2024.
Difference between revisions of "Carbohydrate Binding Module Family 66"
| Line 21: | Line 21: | ||
== Structural Features == | == Structural Features == | ||
| − | [[File:CBM66fold.jpg|thumb|300px|right|'''Figure 1.''' The | + | [[File:CBM66fold.jpg|thumb|300px|right|'''Figure 1.''' The crystal structure of BsCBM60 in complex with levanotriose ([{{PDBlink}}4B1M PDB ID 4B1M]), highlighting the location of the ligand binding site on the concave &beat;sheet (A). B shows the surface representation of the CBM. In the fructose located in the ligand binding site O2 and O6 (both in cyan) are pointing at the surface of the protein and thus the sugar cannot be extended in the non-reducing direction.]] |
| − | The crystal structure of BsCBM66 showed that the protein adopts a β-sandwich fold in which the two β-sheets contain seven and six antiparallel β-strands, respectively. The surface of the protein reveals a broad pocket centered on the concave β-sheet (Figure 1A). In | + | The crystal structure of the apo form of BsCBM66 showed that the protein adopts a β-sandwich fold in which the two β-sheets contain seven and six antiparallel β-strands, respectively ([{{PDBlink}}AZZ PDB ID AZZ]). The surface of the protein reveals a broad pocket centered on the concave β-sheet (Figure 1A). In complex with fructose ([{{PDBlink}}4B1L PDB ID 4B1L]) or levantriose ([{{PDBlink}}4B1M PDB ID 4B1M]) the sugars were located in the broad pocket demonstrating that it comprises the ligand binding site. Fructose, which is in its furanose form, is sandwiched between two aromatic residues, which make non-polar interactions with the ligand. These hydrophobic contacts are augmented by polar interactions with a lysine, asparagine and two aspartates. Mutagenesis showed that all these residues are essential for ligand binding. The O1 and O6 of the bound fructose are pointing at the surface of BsCBM66 (Figure 1B), preventing extension of the ligand in the non-reducing direction (levan and inulin are linked β-2,6– and β-2,1, respectively), whereas O2 is pointing into solvent, consistent with the protein binding to the non-reducing termini of β-2,6– and β-2,1–linked fructans. This confirms the designation of BsCBM66 as a type C CBM. The ligand-binding residues identified above are conserved in 37 CBM66 members, and thus it is likely that these protein modules display specificity for the non-reducing end of levan and inulin and bind through a common mechanism. In the complex with levantriose the non-reducing fructose makes the same interactions with BsCBM66 as fructose. The central and terminal fructose in the trisaccharide do not make biologically significant interactions with the protein. |
== Functionalities == | == Functionalities == | ||
The vast majority of CBM66s are located in GH32 enzymes that target fructans. Based on this observation it was suggested that the predominant role of CBM66 is in binding fructans. The remaining CBM66 members are linked to a range of CAZy enzymes, primarily glycoside hydrolases and lyases, associated with plant cell-wall degradation, although the binding properties of these modules are unknown <cite>Cuskin2012</cite>. Biochemical analysis revealed that SacC displayed ∼100-fold-higher activity for levan (β-2,6–glycosidic linkages) compared with inulin (β-2,1–glycosidic linkages) or oligosaccharides [with a degree of polymerization (DP) <6] of either fructan. Truncation experiments showed that this elevated activity against levan was conferred by BsCBM66 <cite>Cuskin2012</cite>. Fusion of BsCBM66 onto a non-specific fructosidase caused a 100-fold increase in the activity of the enzyme against levan but did influence the activity of the glycoside hydrolase against oligosaccharides or inulin <cite>Cuskin2012</cite>. It was argued that increased activity against levan reflects its highly branched structure compared to inulin. Thus, the BsCBM66 and the GH32 catalytic module within SacC would be able to bind to different terminal fructose residues of the same polysaccharide molecule. It was proposed that the ensuing avidity effect resulted in much tighter binding of SacC to levan, compared to the catalytic module as a discrete entity, leading to increased catalytic activity. It was proposed that the avidity, and thus increase in substrate binding of the levanase, may be greater in the many GH32 enzymes that contain two or more CBM66 modules. It was also suggested that the proposed avidity mechanism for BsCBM66 function may be a generic feature of CBMs that bind to the terminal residues of glycans, and are linked to exo-acting glycanases. Examples cited include GH84 exo-GlcNAcases, which often contain CBM32s that target terminal Gal-GlcNAc structures present on the surface of red blood cells <cite>Ficko-Blean2006</cite>. | The vast majority of CBM66s are located in GH32 enzymes that target fructans. Based on this observation it was suggested that the predominant role of CBM66 is in binding fructans. The remaining CBM66 members are linked to a range of CAZy enzymes, primarily glycoside hydrolases and lyases, associated with plant cell-wall degradation, although the binding properties of these modules are unknown <cite>Cuskin2012</cite>. Biochemical analysis revealed that SacC displayed ∼100-fold-higher activity for levan (β-2,6–glycosidic linkages) compared with inulin (β-2,1–glycosidic linkages) or oligosaccharides [with a degree of polymerization (DP) <6] of either fructan. Truncation experiments showed that this elevated activity against levan was conferred by BsCBM66 <cite>Cuskin2012</cite>. Fusion of BsCBM66 onto a non-specific fructosidase caused a 100-fold increase in the activity of the enzyme against levan but did influence the activity of the glycoside hydrolase against oligosaccharides or inulin <cite>Cuskin2012</cite>. It was argued that increased activity against levan reflects its highly branched structure compared to inulin. Thus, the BsCBM66 and the GH32 catalytic module within SacC would be able to bind to different terminal fructose residues of the same polysaccharide molecule. It was proposed that the ensuing avidity effect resulted in much tighter binding of SacC to levan, compared to the catalytic module as a discrete entity, leading to increased catalytic activity. It was proposed that the avidity, and thus increase in substrate binding of the levanase, may be greater in the many GH32 enzymes that contain two or more CBM66 modules. It was also suggested that the proposed avidity mechanism for BsCBM66 function may be a generic feature of CBMs that bind to the terminal residues of glycans, and are linked to exo-acting glycanases. Examples cited include GH84 exo-GlcNAcases, which often contain CBM32s that target terminal Gal-GlcNAc structures present on the surface of red blood cells <cite>Ficko-Blean2006</cite>. | ||
Revision as of 15:53, 21 January 2018
This page is currently under construction. This means that the Responsible Curator has deemed that the page's content is not quite up to CAZypedia's standards for full public consumption. All information should be considered to be under revision and may be subject to major changes.
- Author: ^^^Harry Gilbert^^^
- Responsible Curator: ^^^Harry Gilbert^^^
| CAZy DB link | |
| http://www.cazy.org/CBM66.html |
Ligand specificities
CBM66 is predominantly a bacterial family that comprise protein modules of around 160 amino acids. The C-terminal module of the Bacillus subtilis exo-levanase (SusC; locus tag BSU27030) is a CBM66 module, defined as BsCBM66, which was shown to bind weakly to fructose (KA ~100 M-1) but with higher affinity to inulin, levan and their respectively oligosaccharides (KA 0.1-1 x 103 M-1) [1]. The affinity of the CBM66 for its ligands was enthalpically driven. To determine whether BsCBM66 recognizes internal or terminal regions of levan, the stoichiometry of binding was compared with that of an inactive form (lacking the catalytic nucleophile) of the exo-acting β-fructosidase BT3082 [2]. The data showed that both proteins bind to Erwinia herbicola levan at the same frequency [1]. It would appear, therefore, that BsCBM66 binds to the non-reducing end of levan chains, a view consistent with the crystal structure of the protein in complex with its target ligands, described below. BsCBM66 is thus a type C CBM.
Structural Features
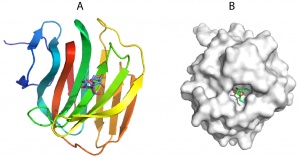
The crystal structure of the apo form of BsCBM66 showed that the protein adopts a β-sandwich fold in which the two β-sheets contain seven and six antiparallel β-strands, respectively (PDB ID AZZ). The surface of the protein reveals a broad pocket centered on the concave β-sheet (Figure 1A). In complex with fructose (PDB ID 4B1L) or levantriose (PDB ID 4B1M) the sugars were located in the broad pocket demonstrating that it comprises the ligand binding site. Fructose, which is in its furanose form, is sandwiched between two aromatic residues, which make non-polar interactions with the ligand. These hydrophobic contacts are augmented by polar interactions with a lysine, asparagine and two aspartates. Mutagenesis showed that all these residues are essential for ligand binding. The O1 and O6 of the bound fructose are pointing at the surface of BsCBM66 (Figure 1B), preventing extension of the ligand in the non-reducing direction (levan and inulin are linked β-2,6– and β-2,1, respectively), whereas O2 is pointing into solvent, consistent with the protein binding to the non-reducing termini of β-2,6– and β-2,1–linked fructans. This confirms the designation of BsCBM66 as a type C CBM. The ligand-binding residues identified above are conserved in 37 CBM66 members, and thus it is likely that these protein modules display specificity for the non-reducing end of levan and inulin and bind through a common mechanism. In the complex with levantriose the non-reducing fructose makes the same interactions with BsCBM66 as fructose. The central and terminal fructose in the trisaccharide do not make biologically significant interactions with the protein.
Functionalities
The vast majority of CBM66s are located in GH32 enzymes that target fructans. Based on this observation it was suggested that the predominant role of CBM66 is in binding fructans. The remaining CBM66 members are linked to a range of CAZy enzymes, primarily glycoside hydrolases and lyases, associated with plant cell-wall degradation, although the binding properties of these modules are unknown [1]. Biochemical analysis revealed that SacC displayed ∼100-fold-higher activity for levan (β-2,6–glycosidic linkages) compared with inulin (β-2,1–glycosidic linkages) or oligosaccharides [with a degree of polymerization (DP) <6] of either fructan. Truncation experiments showed that this elevated activity against levan was conferred by BsCBM66 [1]. Fusion of BsCBM66 onto a non-specific fructosidase caused a 100-fold increase in the activity of the enzyme against levan but did influence the activity of the glycoside hydrolase against oligosaccharides or inulin [1]. It was argued that increased activity against levan reflects its highly branched structure compared to inulin. Thus, the BsCBM66 and the GH32 catalytic module within SacC would be able to bind to different terminal fructose residues of the same polysaccharide molecule. It was proposed that the ensuing avidity effect resulted in much tighter binding of SacC to levan, compared to the catalytic module as a discrete entity, leading to increased catalytic activity. It was proposed that the avidity, and thus increase in substrate binding of the levanase, may be greater in the many GH32 enzymes that contain two or more CBM66 modules. It was also suggested that the proposed avidity mechanism for BsCBM66 function may be a generic feature of CBMs that bind to the terminal residues of glycans, and are linked to exo-acting glycanases. Examples cited include GH84 exo-GlcNAcases, which often contain CBM32s that target terminal Gal-GlcNAc structures present on the surface of red blood cells [3].
Family Firsts
- First Identified
BsCBM66 was the first member of the family to be identified and characterized [1].
- First Structural Characterization
The first structural characterization of a member of family CBM66 was BsCBM66 [1].
References
- Cuskin F, Flint JE, Gloster TM, Morland C, Baslé A, Henrissat B, Coutinho PM, Strazzulli A, Solovyova AS, Davies GJ, and Gilbert HJ. (2012). How nature can exploit nonspecific catalytic and carbohydrate binding modules to create enzymatic specificity. Proc Natl Acad Sci U S A. 2012;109(51):20889-94. DOI:10.1073/pnas.1212034109 |
- Sonnenburg ED, Zheng H, Joglekar P, Higginbottom SK, Firbank SJ, Bolam DN, and Sonnenburg JL. (2010). Specificity of polysaccharide use in intestinal bacteroides species determines diet-induced microbiota alterations. Cell. 2010;141(7):1241-52. DOI:10.1016/j.cell.2010.05.005 |
- Ficko-Blean E and Boraston AB. (2006). The interaction of a carbohydrate-binding module from a Clostridium perfringens N-acetyl-beta-hexosaminidase with its carbohydrate receptor. J Biol Chem. 2006;281(49):37748-57. DOI:10.1074/jbc.M606126200 |