CAZypedia needs your help! We have many unassigned GH, PL, CE, AA, GT, and CBM pages in need of Authors and Responsible Curators.
Scientists at all career stages, including students, are welcome to contribute to CAZypedia. Read more here, and in the 10th anniversary article in Glycobiology.
New to the CAZy classification? Read this first.
*
Consider attending the 15th Carbohydrate Bioengineering Meeting in Ghent, 5-8 May 2024.
Difference between revisions of "Carbohydrate Binding Module Family 78"
| Line 16: | Line 16: | ||
<!-- This is the end of the table --> | <!-- This is the end of the table --> | ||
== Ligand specificities == | == Ligand specificities == | ||
| − | CBM78 is a family identified in the ''Ruminococcus flavefaciens'' cellulosome, a ruminal cellulolytic bacterium <cite>RinconMT2010</cite>. CBM78 | + | CBM78 is a family identified in the ''Ruminococcus flavefaciens'' cellulosome, a ruminal cellulolytic bacterium <cite>RinconMT2010</cite>. The only characterized CBM78 modules are CBM78<sub>RfGH5</sub> and CBM78<sub>RfGH26</sub>, which components of enzymes containing catalytic modules derived from GH5_4 and GH26, respectively. Both CBM78 modules bind to β-1,4- and mixed linked β-1,3-1,4-glucans, however,CBM78<sub>RfGH26</sub>, also binds galactomannan. and contains a GH26 “β1,4-mannanase” catalytic module <cite>VendittoI2016</cite>. CBM78<sub>RfGH5</sub> displays highest affinity for xyloglucan. The similar affinity of CBM78<sub>RfGH5</sub> for cellohexaose and cellopentaose suggests five dominant sugar binding sites. The higher affinity of CBM78<sub>RfGH5</sub> for the xyloglucan derived oligosaccharide XXXG (where X is a backbone glucose decorated with xylose and G is an unsubstituted glucose) than cellotetraose suggests recognition of the xylose side chains. No binding to regenerated (noncrystalline) insoluble cellulose (RC) was detected. |
== Structural Features == | == Structural Features == | ||
[[File:CBM78.jpg|thumb|300px|right|'''Figure 1.''' Crystal structure of CBM78<sub>RfGH5</sub>. ([{{PDBlink}}4VI7 PDB ID 4VI7]). The aromatic residues that contribute to ligand recognition are shown.]] | [[File:CBM78.jpg|thumb|300px|right|'''Figure 1.''' Crystal structure of CBM78<sub>RfGH5</sub>. ([{{PDBlink}}4VI7 PDB ID 4VI7]). The aromatic residues that contribute to ligand recognition are shown.]] | ||
| − | |||
| − | |||
The structure of CBM78<sub>RfGH5</sub> was solved using single-wavelength anomalous diffraction (SAD) methods and selenomethionyl protein to a resolution of 2.0 Å. CBM78<sub>RfGH5</sub> has a β-sandwich fold and contains two β-sheets, 1 and 2, respectively (Figure 1) <cite>VendittoI2016</cite>. β-sheet 2 forms a cleft in which aromatic residues are a dominant feature. Trp496, Trp554, Tyr555, and Phe479 are aligned along the cleft. This hydrophobic region is the glucan binding site in CBM78<sub>RfGH5</sub> <cite>VendittoI2016</cite>. | The structure of CBM78<sub>RfGH5</sub> was solved using single-wavelength anomalous diffraction (SAD) methods and selenomethionyl protein to a resolution of 2.0 Å. CBM78<sub>RfGH5</sub> has a β-sandwich fold and contains two β-sheets, 1 and 2, respectively (Figure 1) <cite>VendittoI2016</cite>. β-sheet 2 forms a cleft in which aromatic residues are a dominant feature. Trp496, Trp554, Tyr555, and Phe479 are aligned along the cleft. This hydrophobic region is the glucan binding site in CBM78<sub>RfGH5</sub> <cite>VendittoI2016</cite>. | ||
== Functionalities == | == Functionalities == | ||
| − | CBM78 plays an enzyme-targeting role that is specific to Ruminococcus and a contribution to enzyme function in a highly complex scaffolding. CBM78 that binds β-glucans is component of enzyme that contains catalytic modules derived from GH5_4 with endo-β1,4-glucanases activity. CBM78 is also component of enzyme that contains catalytic modules derived from GH26 with β1,4-mannanase activity. Mutagenesis experiments confirmed the importance of the aromatic residues in ligand recognition of CBM78<sub>RfGH5</sub>. Alanine substitution of Trp496 or Trp554 in CBM78<sub>RfGH5</sub>, which are conserved in the CBM family, resulted in complete loss of binding to all ligands. The mutants F479A and Y555A bound to xyloglucan, but not to barley β-glucan or HEC.The variant Q552A recognized xyloglucan and barley β-glucan, but not HEC. No binding to regenerated (noncrystalline) insoluble cellulose is detected and it is explained by the narrow binding cleft of CBM78<sub>RfGH5</sub>.The mutagenesis data show that different residues play distinct roles in ligand recognition, explaining why this CBM can bind to a range of β-glucans. | + | CBM78 plays an enzyme-targeting role that is specific to ''Ruminococcus'' and a contribution to enzyme function in a highly complex scaffolding. CBM78 that binds β-glucans is component of enzyme that contains catalytic modules derived from GH5_4 with endo-β1,4-glucanases activity. CBM78 is also component of enzyme that contains catalytic modules derived from GH26 with β1,4-mannanase activity. Mutagenesis experiments confirmed the importance of the aromatic residues in ligand recognition of CBM78<sub>RfGH5</sub>. Alanine substitution of Trp496 or Trp554 in CBM78<sub>RfGH5</sub>, which are conserved in the CBM family, resulted in complete loss of binding to all ligands. The mutants F479A and Y555A bound to xyloglucan, but not to barley β-glucan or HEC.The variant Q552A recognized xyloglucan and barley β-glucan, but not HEC. No binding to regenerated (noncrystalline) insoluble cellulose is detected and it is explained by the narrow binding cleft of CBM78<sub>RfGH5</sub>.The mutagenesis data show that different residues play distinct roles in ligand recognition, explaining why this CBM can bind to a range of β-glucans. |
== Family Firsts == | == Family Firsts == | ||
Revision as of 07:00, 22 February 2018
This page is currently under construction. This means that the Responsible Curator has deemed that the page's content is not quite up to CAZypedia's standards for full public consumption. All information should be considered to be under revision and may be subject to major changes.
- Author: ^^^Immacolata Venditto^^^
- Responsible Curator: ^^^Harry Gilbert^^^
| CAZy DB link | |
| http://www.cazy.org/CBM78.html |
Ligand specificities
CBM78 is a family identified in the Ruminococcus flavefaciens cellulosome, a ruminal cellulolytic bacterium [1]. The only characterized CBM78 modules are CBM78RfGH5 and CBM78RfGH26, which components of enzymes containing catalytic modules derived from GH5_4 and GH26, respectively. Both CBM78 modules bind to β-1,4- and mixed linked β-1,3-1,4-glucans, however,CBM78RfGH26, also binds galactomannan. and contains a GH26 “β1,4-mannanase” catalytic module [2]. CBM78RfGH5 displays highest affinity for xyloglucan. The similar affinity of CBM78RfGH5 for cellohexaose and cellopentaose suggests five dominant sugar binding sites. The higher affinity of CBM78RfGH5 for the xyloglucan derived oligosaccharide XXXG (where X is a backbone glucose decorated with xylose and G is an unsubstituted glucose) than cellotetraose suggests recognition of the xylose side chains. No binding to regenerated (noncrystalline) insoluble cellulose (RC) was detected.
Structural Features
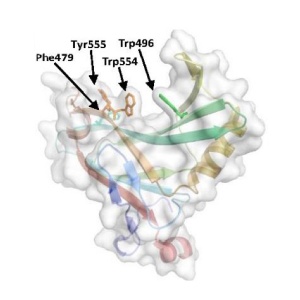
The structure of CBM78RfGH5 was solved using single-wavelength anomalous diffraction (SAD) methods and selenomethionyl protein to a resolution of 2.0 Å. CBM78RfGH5 has a β-sandwich fold and contains two β-sheets, 1 and 2, respectively (Figure 1) [2]. β-sheet 2 forms a cleft in which aromatic residues are a dominant feature. Trp496, Trp554, Tyr555, and Phe479 are aligned along the cleft. This hydrophobic region is the glucan binding site in CBM78RfGH5 [2].
Functionalities
CBM78 plays an enzyme-targeting role that is specific to Ruminococcus and a contribution to enzyme function in a highly complex scaffolding. CBM78 that binds β-glucans is component of enzyme that contains catalytic modules derived from GH5_4 with endo-β1,4-glucanases activity. CBM78 is also component of enzyme that contains catalytic modules derived from GH26 with β1,4-mannanase activity. Mutagenesis experiments confirmed the importance of the aromatic residues in ligand recognition of CBM78RfGH5. Alanine substitution of Trp496 or Trp554 in CBM78RfGH5, which are conserved in the CBM family, resulted in complete loss of binding to all ligands. The mutants F479A and Y555A bound to xyloglucan, but not to barley β-glucan or HEC.The variant Q552A recognized xyloglucan and barley β-glucan, but not HEC. No binding to regenerated (noncrystalline) insoluble cellulose is detected and it is explained by the narrow binding cleft of CBM78RfGH5.The mutagenesis data show that different residues play distinct roles in ligand recognition, explaining why this CBM can bind to a range of β-glucans.
Family Firsts
- First Identified
- CBM78 from the Ruminococcus flavefaciens CBM78RfGH5 and CBM78RfGH26 .
- First Structural Characterization
- The first available crystal structure and the first complex structure of a CBM78 is from CBM78RfGH5.
References
- Rincon MT, Dassa B, Flint HJ, Travis AJ, Jindou S, Borovok I, Lamed R, Bayer EA, Henrissat B, Coutinho PM, Antonopoulos DA, Berg Miller ME, and White BA. (2010). Abundance and diversity of dockerin-containing proteins in the fiber-degrading rumen bacterium, Ruminococcus flavefaciens FD-1. PLoS One. 2010;5(8):e12476. DOI:10.1371/journal.pone.0012476 |
- Venditto I, Luis AS, Rydahl M, Schückel J, Fernandes VO, Vidal-Melgosa S, Bule P, Goyal A, Pires VM, Dourado CG, Ferreira LM, Coutinho PM, Henrissat B, Knox JP, Baslé A, Najmudin S, Gilbert HJ, Willats WG, and Fontes CM. (2016). Complexity of the Ruminococcus flavefaciens cellulosome reflects an expansion in glycan recognition. Proc Natl Acad Sci U S A. 2016;113(26):7136-41. DOI:10.1073/pnas.1601558113 |