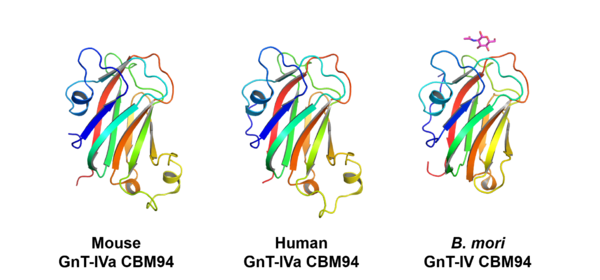CAZypedia needs your help! We have many unassigned GH, PL, CE, AA, GT, and CBM pages in need of Authors and Responsible Curators.
Scientists at all career stages, including students, are welcome to contribute to CAZypedia. Read more here, and in the 10th anniversary article in Glycobiology.
New to the CAZy classification? Read this first.
*
Consider attending the 15th Carbohydrate Bioengineering Meeting in Ghent, 5-8 May 2024.
Difference between revisions of "Carbohydrate Binding Module Family 94"
| Line 21: | Line 21: | ||
CBM94 was established in 2022 after the structural and functional characterization of the C-terminal domains of human ''N''-acetylglucosaminyltransferase IVa (GnT-IVa, MGAT4A; [[GT54]]; EC [{{EClink}}2.4.1.145 2.4.1.145], Fig. 1) and an ortholog from lepidopteran insect ''Bombyx mori'' (silkworm) <cite>Oka2022</cite>. The CBM94 proteins from human and ''B. mori'' showed affinity toward ''N''-acetylglucosamine (GlcNAc), ''N'',''N''’-diacetylchitobiose, and ''p''-nitrophenyl β-''N''-acetylglucosaminide with ''K''<sub>a</sub> values of 242–1970 M<sup>−1</sup>. No affinity was detected for other monosaccharides, including glucose, mannose, galactose, L-fucose, and ''N''-acetylgalactosamine, some of which are components of matured ''N''-glycans <cite>Oka2022</cite>. Nagae et al. demonstrated that the C-terminal domain of mouse GnT-IVa has binding ability for GlcNAc and GlcNAc-β-(1→2)-Man using NMR titration analysis <cite>Nagae2022</cite>. Furthermore, comprehensive frontal affinity chromatography analysis using 157 glycans showed that mouse CBM94 has affinity for ''N''-glycans with β-(1→2) and β-(1→4)-linked GlcNAc at the non-reducing ends. On the other hand, it showed lower affinity for ''N''-glycan with only β-(1→2)-linked GlcNAc, which is the substrate of GnT-IV <cite>Nagae2022</cite>. Therefore, GnT-IVa CBM94 prefers product ''N''-glycans to substrate ''N''-glycans (Fig. 1). | CBM94 was established in 2022 after the structural and functional characterization of the C-terminal domains of human ''N''-acetylglucosaminyltransferase IVa (GnT-IVa, MGAT4A; [[GT54]]; EC [{{EClink}}2.4.1.145 2.4.1.145], Fig. 1) and an ortholog from lepidopteran insect ''Bombyx mori'' (silkworm) <cite>Oka2022</cite>. The CBM94 proteins from human and ''B. mori'' showed affinity toward ''N''-acetylglucosamine (GlcNAc), ''N'',''N''’-diacetylchitobiose, and ''p''-nitrophenyl β-''N''-acetylglucosaminide with ''K''<sub>a</sub> values of 242–1970 M<sup>−1</sup>. No affinity was detected for other monosaccharides, including glucose, mannose, galactose, L-fucose, and ''N''-acetylgalactosamine, some of which are components of matured ''N''-glycans <cite>Oka2022</cite>. Nagae et al. demonstrated that the C-terminal domain of mouse GnT-IVa has binding ability for GlcNAc and GlcNAc-β-(1→2)-Man using NMR titration analysis <cite>Nagae2022</cite>. Furthermore, comprehensive frontal affinity chromatography analysis using 157 glycans showed that mouse CBM94 has affinity for ''N''-glycans with β-(1→2) and β-(1→4)-linked GlcNAc at the non-reducing ends. On the other hand, it showed lower affinity for ''N''-glycan with only β-(1→2)-linked GlcNAc, which is the substrate of GnT-IV <cite>Nagae2022</cite>. Therefore, GnT-IVa CBM94 prefers product ''N''-glycans to substrate ''N''-glycans (Fig. 1). | ||
== Structural Features == | == Structural Features == | ||
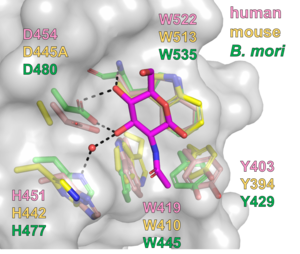
| − | CBM94 domains of GnT-IV enzymes comprise of around 150 amino acid residues. The crystal structures of the CBM94 domains in human and mouse GnT-IVa and ''B. mori'' ortholog were determined at 1.97, 1.95, and 1.47 Å resolution (PDB ID [{{PDBlink}}7XTL 7XTL], [{{PDBlink}}7VMT 7VMT], and [{{PDBlink}}7XTM 7XTM]), respectively. The mammalian CBM94 proteins adopt a β-sandwich fold comprising nine β-strands and three short α-helices, while ''B. mori'' CBM94 has a similar fold but lacks one α-helix (Fig. 2). They are structurally homologous to the [[CBM32]]s, such as the GlcNAc-binding [[CBM32]] domain (NagHCBM32-2) of ''Clostridium perfringens'' [[GH84]] β-''N''-acetylglucosaminidase NagH <cite>Ficko-Blean2009</cite>. The 1.15-Å resolution structure of ''B. mori'' CBM94 in complex with β-GlcNAc (PDB ID [{{PDBlink}}7XTN 7XTN]) indicates that Tyr429, Trp445, Asp480, and Trp535 contribute to GlcNAc binding (Fig. 3). These residues are completely conserved among NagHCBM32-2 and CBM94 domains in mammalian GnT-IV isozymes (GnT-IVa, GnT-IVb, and GnT-IVc) except that Tyr429 is substituted to Phe in GnT-IVc. | + | CBM94 domains of GnT-IV enzymes comprise of around 150 amino acid residues. The crystal structures of the CBM94 domains in human and mouse GnT-IVa and ''B. mori'' ortholog were determined at 1.97, 1.95, and 1.47 Å resolution (PDB ID [{{PDBlink}}7XTL 7XTL], [{{PDBlink}}7VMT 7VMT], and [{{PDBlink}}7XTM 7XTM]), respectively. The mammalian CBM94 proteins adopt a β-sandwich fold comprising nine β-strands and three short α-helices, while ''B. mori'' CBM94 has a similar fold but lacks one α-helix (Fig. 2). They are structurally homologous to the [[CBM32]]s, such as the GlcNAc-binding [[CBM32]] domain (NagHCBM32-2) of the ''Clostridium perfringens'' [[GH84]] β-''N''-acetylglucosaminidase NagH <cite>Ficko-Blean2009</cite>. The 1.15-Å resolution structure of ''B. mori'' CBM94 in complex with β-GlcNAc (PDB ID [{{PDBlink}}7XTN 7XTN]) indicates that Tyr429, Trp445, Asp480, and Trp535 contribute to GlcNAc binding (Fig. 3). These residues are completely conserved among NagHCBM32-2 and CBM94 domains in mammalian GnT-IV isozymes (GnT-IVa, GnT-IVb, and GnT-IVc) except that Tyr429 is substituted to Phe in GnT-IVc. |
[[File:Fig2 CBM94 structures.png|thumb|600px|center|'''Figure 2. Overall structures of CBM94 proteins.''' (Left to right) Mouse GnT-IVa CBM94 (D445A mutant) [{{PDBlink}}7VMT 7VMT], human GnT-IVa CBM94 [{{PDBlink}}7XTL 7XTL], and ''B. mori'' GnT-IV ortholog CBM94 in complex with GlcNAc (''magenta'' stick) [{{PDBlink}}7XTN 7XTN]. ]] | [[File:Fig2 CBM94 structures.png|thumb|600px|center|'''Figure 2. Overall structures of CBM94 proteins.''' (Left to right) Mouse GnT-IVa CBM94 (D445A mutant) [{{PDBlink}}7VMT 7VMT], human GnT-IVa CBM94 [{{PDBlink}}7XTL 7XTL], and ''B. mori'' GnT-IV ortholog CBM94 in complex with GlcNAc (''magenta'' stick) [{{PDBlink}}7XTN 7XTN]. ]] | ||
Revision as of 01:49, 13 April 2023
This page has been approved by the Responsible Curator as essentially complete. CAZypedia is a living document, so further improvement of this page is still possible. If you would like to suggest an addition or correction, please contact the page's Responsible Curator directly by e-mail.
| CAZy DB link | |
| http://www.cazy.org/CBM94.html |
Ligand specificities
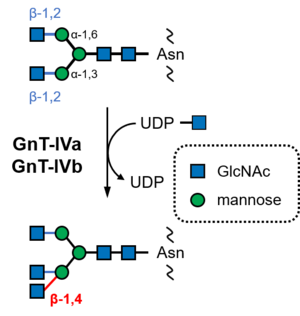
CBM94 was established in 2022 after the structural and functional characterization of the C-terminal domains of human N-acetylglucosaminyltransferase IVa (GnT-IVa, MGAT4A; GT54; EC 2.4.1.145, Fig. 1) and an ortholog from lepidopteran insect Bombyx mori (silkworm) [1]. The CBM94 proteins from human and B. mori showed affinity toward N-acetylglucosamine (GlcNAc), N,N’-diacetylchitobiose, and p-nitrophenyl β-N-acetylglucosaminide with Ka values of 242–1970 M−1. No affinity was detected for other monosaccharides, including glucose, mannose, galactose, L-fucose, and N-acetylgalactosamine, some of which are components of matured N-glycans [1]. Nagae et al. demonstrated that the C-terminal domain of mouse GnT-IVa has binding ability for GlcNAc and GlcNAc-β-(1→2)-Man using NMR titration analysis [2]. Furthermore, comprehensive frontal affinity chromatography analysis using 157 glycans showed that mouse CBM94 has affinity for N-glycans with β-(1→2) and β-(1→4)-linked GlcNAc at the non-reducing ends. On the other hand, it showed lower affinity for N-glycan with only β-(1→2)-linked GlcNAc, which is the substrate of GnT-IV [2]. Therefore, GnT-IVa CBM94 prefers product N-glycans to substrate N-glycans (Fig. 1).
Structural Features
CBM94 domains of GnT-IV enzymes comprise of around 150 amino acid residues. The crystal structures of the CBM94 domains in human and mouse GnT-IVa and B. mori ortholog were determined at 1.97, 1.95, and 1.47 Å resolution (PDB ID 7XTL, 7VMT, and 7XTM), respectively. The mammalian CBM94 proteins adopt a β-sandwich fold comprising nine β-strands and three short α-helices, while B. mori CBM94 has a similar fold but lacks one α-helix (Fig. 2). They are structurally homologous to the CBM32s, such as the GlcNAc-binding CBM32 domain (NagHCBM32-2) of the Clostridium perfringens GH84 β-N-acetylglucosaminidase NagH [3]. The 1.15-Å resolution structure of B. mori CBM94 in complex with β-GlcNAc (PDB ID 7XTN) indicates that Tyr429, Trp445, Asp480, and Trp535 contribute to GlcNAc binding (Fig. 3). These residues are completely conserved among NagHCBM32-2 and CBM94 domains in mammalian GnT-IV isozymes (GnT-IVa, GnT-IVb, and GnT-IVc) except that Tyr429 is substituted to Phe in GnT-IVc.
Functionalities
The CBM94 domains of human GnT-IVa and B. mori ortholog were only examined for affinity to sugars, but the CBM94 domain of mouse GnT-IVa was examined for its relevance to enzyme activity and substrate specificity. The deletion of the CBM94 domain markedly reduced the activity of mouse GnT-IVa, and the replacement of Asp445, which binds GlcNAc, with Ala also reduced the glycosyltransferase activity. Based on its affinity for glycans, it is possible that the function of the CBM94 domain is to regulate the catalytic cycle from enzymatic reaction to product release rather than to capture substrates [2]. A comparative study of mouse GnT-IVa and GnT-IVb suggested that their CBM94 domains affect substrate glycoprotein preference in addition to the glycan binding function [4]. It should be noted that a CBM94 domain is conserved among GnT-IV isozymes, GnT-IVa, -IVb, and -IVc, but is completely absent in GnT-IVd (MGAT4D), which has no enzymatic activity observed and inhibits GnT-I activity [5].
Family Firsts
- First Identified
- Sugar-binding ability of the C-terminal domains of human and mouse GnT-IVa (MGAT4A) and Bombyx mori ortholog was identified independently by two groups [1, 2].
- First Structural Characterization
- Crystal structures of the C-terminal domains of human and mouse GnT-IVa (MGAT4A) and Bombyx mori ortholog were determined independently by two groups [1, 2]. β-GlcNAc-bound structure of B. mori CBM94 was also determined [1].
References
- Oka N, Mori S, Ikegaya M, Park EY, and Miyazaki T. (2022). Crystal structure and sugar-binding ability of the C-terminal domain of N-acetylglucosaminyltransferase IV establish a new carbohydrate-binding module family. Glycobiology. 2022;32(12):1153-1163. DOI:10.1093/glycob/cwac058 |
- Nagae M, Hirata T, Tateno H, Mishra SK, Manabe N, Osada N, Tokoro Y, Yamaguchi Y, Doerksen RJ, Shimizu T, and Kizuka Y. (2022). Discovery of a lectin domain that regulates enzyme activity in mouse N-acetylglucosaminyltransferase-IVa (MGAT4A). Commun Biol. 2022;5(1):695. DOI:10.1038/s42003-022-03661-w |
- Ficko-Blean E and Boraston AB. (2009). N-acetylglucosamine recognition by a family 32 carbohydrate-binding module from Clostridium perfringens NagH. J Mol Biol. 2009;390(2):208-20. DOI:10.1016/j.jmb.2009.04.066 |
- Osada N, Nagae M, Nakano M, Hirata T, and Kizuka Y. (2022). Examination of differential glycoprotein preferences of N-acetylglucosaminyltransferase-IV isozymes a and b. J Biol Chem. 2022;298(9):102400. DOI:10.1016/j.jbc.2022.102400 |
- Huang HH, Hassinen A, Sundaram S, Spiess AN, Kellokumpu S, and Stanley P. (2015). GnT1IP-L specifically inhibits MGAT1 in the Golgi via its luminal domain. Elife. 2015;4. DOI:10.7554/eLife.08916 |