CAZypedia needs your help! We have many unassigned GH, PL, CE, AA, GT, and CBM pages in need of Authors and Responsible Curators.
Scientists at all career stages, including students, are welcome to contribute to CAZypedia. Read more here, and in the 10th anniversary article in Glycobiology.
New to the CAZy classification? Read this first.
*
Consider attending the 15th Carbohydrate Bioengineering Meeting in Ghent, 5-8 May 2024.
Difference between revisions of "File:CAH Bsubtilis CE7.jpg"
Joel Weadge (talk | contribs) (Figure 1. CAH from B. subtilis. A. An individual monomer from the overall CAH structure (PDB ID 1ODS) showing the a/bhydrolase fold formed by each subunit and the b-strand-like interface domain needed for association of the monomers. The catalytic tria...) |
Joel Weadge (talk | contribs) |
||
| Line 1: | Line 1: | ||
| − | Figure 1. CAH from B. subtilis. A. An individual monomer from the overall CAH structure (PDB ID 1ODS) showing the | + | Figure 1. CAH from B. subtilis. A. An individual monomer from the overall CAH structure (PDB ID 1ODS) showing the α/β-hydrolase fold formed by each subunit and the β-strand-like interface domain needed for association of the monomers. The catalytic triad of Ser181, Asp 269 and His 298 are highlighted in cyan. B. Characteristic donut-shaped quaternary structure of the CAH hexamer. Each monomer is represented in a different colour. |
Revision as of 11:54, 22 July 2020
Figure 1. CAH from B. subtilis. A. An individual monomer from the overall CAH structure (PDB ID 1ODS) showing the α/β-hydrolase fold formed by each subunit and the β-strand-like interface domain needed for association of the monomers. The catalytic triad of Ser181, Asp 269 and His 298 are highlighted in cyan. B. Characteristic donut-shaped quaternary structure of the CAH hexamer. Each monomer is represented in a different colour.
File history
Click on a date/time to view the file as it appeared at that time.
| Date/Time | Thumbnail | Dimensions | User | Comment | |
|---|---|---|---|---|---|
| current | 11:53, 22 July 2020 | 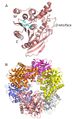 | 745 × 1,110 (178 KB) | Joel Weadge (talk | contribs) | Figure 1. CAH from B. subtilis. A. An individual monomer from the overall CAH structure (PDB ID 1ODS) showing the a/bhydrolase fold formed by each subunit and the b-strand-like interface domain needed for association of the monomers. The catalytic tria... |
You cannot overwrite this file.
File usage
The following page uses this file: