CAZypedia needs your help! We have many unassigned GH, PL, CE, AA, GT, and CBM pages in need of Authors and Responsible Curators.
Scientists at all career stages, including students, are welcome to contribute to CAZypedia. Read more here, and in the 10th anniversary article in Glycobiology.
New to the CAZy classification? Read this first.
*
Consider attending the 15th Carbohydrate Bioengineering Meeting in Ghent, 5-8 May 2024.
Difference between revisions of "Glycoside Hydrolase Family 103"
Harry Brumer (talk | contribs) m |
|||
| Line 26: | Line 26: | ||
</div> | </div> | ||
<!-- This is the end of the table --> | <!-- This is the end of the table --> | ||
| + | |||
== Substrate specificities == | == Substrate specificities == | ||
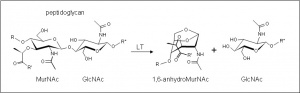
| − | + | [[Image:LTreaction.jpg|thumb|right|'''Figure 1.''' Reaction catalyzed by family GH103 enzymes. LT, lytic transglycosylase. (''click to enlarge'').]]The glycoside hydrolases of this family are lytic transglyosylases (also referred to as peptidoglycan lyases) of bacterial origin and they constitute family 3 of the classification scheme of Blackburn and Clarke <cite>1</cite>. The prototype for this family is membrane-bound lytic transglycosylase B (MltB) from ''Escherichia coli'' <cite>2</cite>. These enzymes cleave the β-1,4 linkage between ''N''-acetylmuramoyl and ''N''-acetylglucosaminyl residues in peptidoglycan (Figure 1), No other activities have been observed. | |
| − | |||
| − | |||
| − | |||
== Kinetics and Mechanism == | == Kinetics and Mechanism == | ||
| − | + | The lytic transglycosidases, strictly speaking, are retaining enzymes. However, they are not hydrolases but rather catalyse an intramolecular glycosyl transferase reaction onto the C-6 hydroxyl group of the muramoyl residue leading to the generation of a terminal 1,6-anhdyromuramoyl product (Figure 1) thus lacking a reducing end <cite>3</cite>. No detailed analyses involving both steady state and pre-steady state kinetic studies have been reported, but the Michaelis Menten (''K''<sub>M</sub> and ''V''<sub>max</sub>) parameters have been estimated for ''Pseudomonas aeruginosa'' MltB acting on insoluble peptidoglycan sacculi <cite>4</cite>. | |
== Catalytic Residues == | == Catalytic Residues == | ||
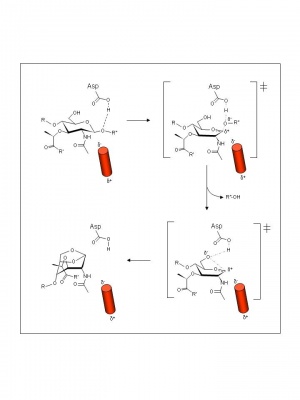
| − | + | [[Image:Mltamechanism.jpg|thumb|right|'''Figure 2.''' Reaction mechanism proposed for ''E. coli'' MltB. (''click to enlarge'').]]As with other lytic transglycosylases (families [[GH23]], [[GH102]], and [[GH104]]), the GH103 enzymes are thought to possess a single catalytic acid/base residue. This residue has been identified as Glu162 in MltB from both ''E. coli'' and ''P. aeruginosa'' and, indeed, its replacement abolishes catalytic activity <cite>4 5</cite>. The mechanism of action of family GH103 enzymes has been investigated the most compared to the lytic transglycosylases of the other families ([[GH23]],[[GH102]], and [[GH104]]). | |
| + | |||
| + | Examination of crystal structures of ''E. coli'' Slt35 (a soluble proteolytic derivative of MltB)and theoretical considerations led to the proposal of a mechanism that accommodates a single catalytic at its active site. Thus, based on the complexes formed with murodipeptide, chitobiose, and the inhibitor bulgecin, a substrate-assisted mechanism, analogous to the family [[GH18]] chitinases and chitobiases, family [[GH20]] ''N''-acetyl-β-hexosaminidases, and family [[GH23]] lytic transglycosylases, has been invoked <cite>6</cite>. Thus, the catalytic Glu162 is proposed to serve initially as an acid catalyst to donate a proton to the glycosidic oxygen of the linkage to be cleaved leading to the formation of an intermediate with oxocarbenium ion character (Figure 2). In the absence of an anion/nucleophile in close proximity to stabilize this oxocarbenium intermediate, the lytic transglycosylases would employ anchimeric assistance of the MurNAc 2-acetamido group resulting in the formation an oxazolinium ion intermediate. This would be followed by abstraction of the C-6 hydroxyl proton of the oxazolinium species involving Glu73 which now serves as the base catalyst leading to nucleophilic attack and the formation of 1,6-anhydromuramic acid product. The β-hexosaminidase inhibitor NAG-thiazoline was found to inhibit ''P. aeruginosa'' MltB thus supporting the proposal for the formation of an oxazolinium ion intermediate <cite>7</cite> | ||
| Line 47: | Line 47: | ||
== Family Firsts == | == Family Firsts == | ||
| − | |||
| − | |||
| − | |||
| − | |||
| + | == Family Firsts == | ||
| + | ;First identification of lytic transglycosylase: MltA from ''E. coli'' <cite>2</cite>. | ||
| + | ;First catalytic nucleophile identification: Not applicable for lytic transglycosylases. | ||
| + | ;First general acid/base residue identification: Inferred by X-ray crystallography of ''E. coli'' MltA <cite>4</cite>. | ||
| + | ;First 3-D structure: ''E. coli'' MltA <cite>4</cite>. | ||
| + | ;First identification as a lipoprotein: ''E. coli'' MltA <cite>7</cite>. | ||
| + | ;First identification of localization to outer membrane: ''E. coli'' MltA <cite>7</cite>. | ||
| + | ;Frist demonstration of molecular interactions between GH102 enzymes and penicillin-binding proteins:''E. coli'' MltA <cite>8</cite>. | ||
== References == | == References == | ||
<biblio> | <biblio> | ||
| − | # | + | #1 pmid=11139297 |
| − | # | + | #2 pmid=1356966 |
| − | #3 | + | #3 pmid=357 |
| − | # | + | #4 pmid=11790124 |
| − | + | #5 pmid=10545329 | |
| + | #6 pmid=10684641 | ||
| + | #7 pmid=15358542 | ||
| + | #8 pmid= | ||
| + | #9 pmid= | ||
| + | #10 pmid= | ||
| + | #11 pmid= | ||
| + | #12 pmid= | ||
| + | #13 pmid= | ||
</biblio> | </biblio> | ||
[[Category:Glycoside Hydrolase Families|GH103]] | [[Category:Glycoside Hydrolase Families|GH103]] | ||
Revision as of 19:12, 19 February 2010
This page is currently under construction. This means that the Responsible Curator has deemed that the page's content is not quite up to CAZypedia's standards for full public consumption. All information should be considered to be under revision and may be subject to major changes.
- Author: ^^^Anthony Clarke^^^
- Responsible Curator: ^^^Anthony Clarke^^^
| Glycoside Hydrolase Family GHnn | |
| Clan | GH-x |
| Mechanism | retaining/inverting |
| Active site residues | known/not known |
| CAZy DB link | |
| http://www.cazy.org/fam/GHnn.html | |
Substrate specificities
The glycoside hydrolases of this family are lytic transglyosylases (also referred to as peptidoglycan lyases) of bacterial origin and they constitute family 3 of the classification scheme of Blackburn and Clarke [1]. The prototype for this family is membrane-bound lytic transglycosylase B (MltB) from Escherichia coli [2]. These enzymes cleave the β-1,4 linkage between N-acetylmuramoyl and N-acetylglucosaminyl residues in peptidoglycan (Figure 1), No other activities have been observed.
Kinetics and Mechanism
The lytic transglycosidases, strictly speaking, are retaining enzymes. However, they are not hydrolases but rather catalyse an intramolecular glycosyl transferase reaction onto the C-6 hydroxyl group of the muramoyl residue leading to the generation of a terminal 1,6-anhdyromuramoyl product (Figure 1) thus lacking a reducing end [3]. No detailed analyses involving both steady state and pre-steady state kinetic studies have been reported, but the Michaelis Menten (KM and Vmax) parameters have been estimated for Pseudomonas aeruginosa MltB acting on insoluble peptidoglycan sacculi [4].
Catalytic Residues
As with other lytic transglycosylases (families GH23, GH102, and GH104), the GH103 enzymes are thought to possess a single catalytic acid/base residue. This residue has been identified as Glu162 in MltB from both E. coli and P. aeruginosa and, indeed, its replacement abolishes catalytic activity [4, 5]. The mechanism of action of family GH103 enzymes has been investigated the most compared to the lytic transglycosylases of the other families (GH23,GH102, and GH104).
Examination of crystal structures of E. coli Slt35 (a soluble proteolytic derivative of MltB)and theoretical considerations led to the proposal of a mechanism that accommodates a single catalytic at its active site. Thus, based on the complexes formed with murodipeptide, chitobiose, and the inhibitor bulgecin, a substrate-assisted mechanism, analogous to the family GH18 chitinases and chitobiases, family GH20 N-acetyl-β-hexosaminidases, and family GH23 lytic transglycosylases, has been invoked [6]. Thus, the catalytic Glu162 is proposed to serve initially as an acid catalyst to donate a proton to the glycosidic oxygen of the linkage to be cleaved leading to the formation of an intermediate with oxocarbenium ion character (Figure 2). In the absence of an anion/nucleophile in close proximity to stabilize this oxocarbenium intermediate, the lytic transglycosylases would employ anchimeric assistance of the MurNAc 2-acetamido group resulting in the formation an oxazolinium ion intermediate. This would be followed by abstraction of the C-6 hydroxyl proton of the oxazolinium species involving Glu73 which now serves as the base catalyst leading to nucleophilic attack and the formation of 1,6-anhydromuramic acid product. The β-hexosaminidase inhibitor NAG-thiazoline was found to inhibit P. aeruginosa MltB thus supporting the proposal for the formation of an oxazolinium ion intermediate [7]
Three-dimensional structures
Content is to be added here.
Family Firsts
Family Firsts
- First identification of lytic transglycosylase
- MltA from E. coli [2].
- First catalytic nucleophile identification
- Not applicable for lytic transglycosylases.
- First general acid/base residue identification
- Inferred by X-ray crystallography of E. coli MltA [4].
- First 3-D structure
- E. coli MltA [4].
- First identification as a lipoprotein
- E. coli MltA [7].
- First identification of localization to outer membrane
- E. coli MltA [7].
- Frist demonstration of molecular interactions between GH102 enzymes and penicillin-binding proteins
- E. coli MltA [8].
References
- Blackburn NT and Clarke AJ. (2001). Identification of four families of peptidoglycan lytic transglycosylases. J Mol Evol. 2001;52(1):78-84. DOI:10.1007/s002390010136 |
- Engel H, Smink AJ, van Wijngaarden L, and Keck W. (1992). Murein-metabolizing enzymes from Escherichia coli: existence of a second lytic transglycosylase. J Bacteriol. 1992;174(20):6394-403. DOI:10.1128/jb.174.20.6394-6403.1992 |
- Höltje JV, Mirelman D, Sharon N, and Schwarz U. (1975). Novel type of murein transglycosylase in Escherichia coli. J Bacteriol. 1975;124(3):1067-76. DOI:10.1128/jb.124.3.1067-1076.1975 |
- Blackburn NT and Clarke AJ. (2002). Characterization of soluble and membrane-bound family 3 lytic transglycosylases from Pseudomonas aeruginosa. Biochemistry. 2002;41(3):1001-13. DOI:10.1021/bi011833k |
- van Asselt EJ, Dijkstra AJ, Kalk KH, Takacs B, Keck W, and Dijkstra BW. (1999). Crystal structure of Escherichia coli lytic transglycosylase Slt35 reveals a lysozyme-like catalytic domain with an EF-hand. Structure. 1999;7(10):1167-80. DOI:10.1016/s0969-2126(00)80051-9 |
- van Asselt EJ, Kalk KH, and Dijkstra BW. (2000). Crystallographic studies of the interactions of Escherichia coli lytic transglycosylase Slt35 with peptidoglycan. Biochemistry. 2000;39(8):1924-34. DOI:10.1021/bi992161p |
- Reid CW, Blackburn NT, Legaree BA, Auzanneau FI, and Clarke AJ. (2004). Inhibition of membrane-bound lytic transglycosylase B by NAG-thiazoline. FEBS Lett. 2004;574(1-3):73-9. DOI:10.1016/j.febslet.2004.08.006 |
-
pmid=
-
pmid=
-
pmid=
-
pmid=
-
pmid=
-
pmid=