CAZypedia needs your help! We have many unassigned GH, PL, CE, AA, GT, and CBM pages in need of Authors and Responsible Curators.
Scientists at all career stages, including students, are welcome to contribute to CAZypedia. Read more here, and in the 10th anniversary article in Glycobiology.
New to the CAZy classification? Read this first.
*
Consider attending the 15th Carbohydrate Bioengineering Meeting in Ghent, 5-8 May 2024.
Difference between revisions of "Glycoside Hydrolase Family 104"
Harry Brumer (talk | contribs) m (updated CAZyDBlink) |
Harry Brumer (talk | contribs) m (increased figure size) |
||
| Line 29: | Line 29: | ||
== Substrate specificities == | == Substrate specificities == | ||
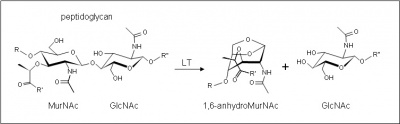
| − | [[Image:LTreaction.jpg|thumb|right|'''Figure 1.''' Reaction catalyzed by family GH104 enzymes. LT, lytic transglycosylase | + | [[Image:LTreaction.jpg|thumb|400px|right|'''Figure 1.''' Reaction catalyzed by family GH104 enzymes. LT, lytic transglycosylase.]]The [[glycoside hydrolases]] of this family are lytic transglyosylases (also referred to as peptidoglycan lyases) of bacteriophage origin and they constitute family 4 of the classification scheme of Blackburn and Clarke <cite>1</cite>. Unfortunately, the origin of many of the hypothetical enzymes listed in GH104 is misleading because they are encoded within prophages which have been integrated into the chromosome of their bacterial host. In other cases, the phage enzyme has been acquired by pathogenicity islands on the bacterial chromosome. The prototype for this family is the enzyme from ''lambda'' phage. |
These enzymes cleave the β-1,4 linkage between ''N''-acetylmuramoyl and ''N''-acetylglucosaminyl residues in peptidoglycan (Figure 1). No other activities have been observed. | These enzymes cleave the β-1,4 linkage between ''N''-acetylmuramoyl and ''N''-acetylglucosaminyl residues in peptidoglycan (Figure 1). No other activities have been observed. | ||
Revision as of 12:47, 26 August 2013
This page has been approved by the Responsible Curator as essentially complete. CAZypedia is a living document, so further improvement of this page is still possible. If you would like to suggest an addition or correction, please contact the page's Responsible Curator directly by e-mail.
- Author: ^^^Anthony Clarke^^^
- Responsible Curator: ^^^Anthony Clarke^^^
| Glycoside Hydrolase Family GH104 | |
| Clan | none
α+β “lysozyme fold |
| Mechanism | retaining |
| Active site residues | known |
| CAZy DB link | |
| http://www.cazy.org/GH104.html | |
Substrate specificities
The glycoside hydrolases of this family are lytic transglyosylases (also referred to as peptidoglycan lyases) of bacteriophage origin and they constitute family 4 of the classification scheme of Blackburn and Clarke [1]. Unfortunately, the origin of many of the hypothetical enzymes listed in GH104 is misleading because they are encoded within prophages which have been integrated into the chromosome of their bacterial host. In other cases, the phage enzyme has been acquired by pathogenicity islands on the bacterial chromosome. The prototype for this family is the enzyme from lambda phage.
These enzymes cleave the β-1,4 linkage between N-acetylmuramoyl and N-acetylglucosaminyl residues in peptidoglycan (Figure 1). No other activities have been observed.
Kinetics and Mechanism
The lytic transglycosidases, strictly speaking, are retaining enzymes. However, they are not hydrolases but rather catalyse an intramolecular glycosyl transferase reaction onto the C-6 hydroxyl group of the muramoyl residue leading to the generation of a terminal 1,6-anhydromuramoyl product (Figure 1) thus lacking a reducing end [2]. No detailed analyses involving either steady state or pre-steady state kinetic studies have been reported.
Catalytic Residues
As with other lytic transglycosylases (families GH23, GH102, and GH103), the GH104 enzymes are thought to possess a single catalytic general acid/base residue. This residue in lambda bacteriophage lytic transglycosylase has been inferred as Glu19 by X-ray crystallography of the enzyme alone [3] and in complex with two chitohexaose oligomers [4]. The role of an essential carboxyl group in the enzyme was confirmed by chemical modification [4], thus supporting the identification of Glu19. The mechanism of action of the family GH104 enzymes has not been investigated and hence it is not known if they follow that of the lytic transglycosylases of families GH23, GH102, or GH103.
Three-dimensional structures
The three-dimensional structure of only the lambda phage enzyme has been solved [3, 4], and like the other lytic transglycosylases of families GH23, and GH103, it possesses the well characterized α+β “lysozyme fold.”
Family Firsts
- First identification of lytic transglycosylase
- Bacteriophage lambda [3].
- First catalytic nucleophile identification
- Not applicable for lytic transglycosylases.
- First general acid/base residue identification
- Inferred by X-ray crystallography of lambda bacteriophage [3].
- First 3-D structure
- Lambda bacteriophage [3].
References
- Blackburn NT and Clarke AJ. (2001). Identification of four families of peptidoglycan lytic transglycosylases. J Mol Evol. 2001;52(1):78-84. DOI:10.1007/s002390010136 |
- Höltje JV, Mirelman D, Sharon N, and Schwarz U. (1975). Novel type of murein transglycosylase in Escherichia coli. J Bacteriol. 1975;124(3):1067-76. DOI:10.1128/jb.124.3.1067-1076.1975 |
- Evrard C, Fastrez J, and Declercq JP. (1998). Crystal structure of the lysozyme from bacteriophage lambda and its relationship with V and C-type lysozymes. J Mol Biol. 1998;276(1):151-64. DOI:10.1006/jmbi.1997.1499 |
- Leung AK, Duewel HS, Honek JF, and Berghuis AM. (2001). Crystal structure of the lytic transglycosylase from bacteriophage lambda in complex with hexa-N-acetylchitohexaose. Biochemistry. 2001;40(19):5665-73. DOI:10.1021/bi0028035 |