CAZypedia needs your help! We have many unassigned GH, PL, CE, AA, GT, and CBM pages in need of Authors and Responsible Curators.
Scientists at all career stages, including students, are welcome to contribute to CAZypedia. Read more here, and in the 10th anniversary article in Glycobiology.
New to the CAZy classification? Read this first.
*
Consider attending the 15th Carbohydrate Bioengineering Meeting in Ghent, 5-8 May 2024.
Difference between revisions of "Glycoside Hydrolase Family 107"
David Teze (talk | contribs) |
David Teze (talk | contribs) |
||
| Line 33: | Line 33: | ||
== Kinetics and Mechanism == | == Kinetics and Mechanism == | ||
The mechanism was proven to be [[retaining]] by observation of the formation of an alpha-linked mercaptoethanol by transglycosylation.<cite>Vickers2018</cite> It should then follow a [[classical Koshland double-displacement mechanism]] similarly to [[GH29]]. | The mechanism was proven to be [[retaining]] by observation of the formation of an alpha-linked mercaptoethanol by transglycosylation.<cite>Vickers2018</cite> It should then follow a [[classical Koshland double-displacement mechanism]] similarly to [[GH29]]. | ||
| + | [[Image:20191213_Gh107.png|thumb|left|600px|Figure 1: Mechanism of GH107 family. According to <cite>Vickers2018</cite>.]] | ||
| + | |||
| + | <br style="clear: both" /> | ||
| + | |||
== Catalytic Residues == | == Catalytic Residues == | ||
The catalytic nucleophile is an aspartate, while the catalytic acid-base is a histidine. The later is unusual in GHs, and a divergence from [[GH29]], but is likely necessary to avoid electronic repulsion with the substrate sulfate groups. These two residues have been identified by structural superimposition with GH29 enzymes, and are conserved within the few members of the GH107 family. The catalytic His has been confirmed by the lack of activity of th H294Q mutant of ''Mariniflexile fucanivorans'', despite its structure was maintained.<cite>Vickers2018</cite> | The catalytic nucleophile is an aspartate, while the catalytic acid-base is a histidine. The later is unusual in GHs, and a divergence from [[GH29]], but is likely necessary to avoid electronic repulsion with the substrate sulfate groups. These two residues have been identified by structural superimposition with GH29 enzymes, and are conserved within the few members of the GH107 family. The catalytic His has been confirmed by the lack of activity of th H294Q mutant of ''Mariniflexile fucanivorans'', despite its structure was maintained.<cite>Vickers2018</cite> | ||
Revision as of 06:06, 13 December 2019
This page is currently under construction. This means that the Responsible Curator has deemed that the page's content is not quite up to CAZypedia's standards for full public consumption. All information should be considered to be under revision and may be subject to major changes.
- Author: ^^^David Teze^^^
- Responsible Curator: ^^^Al Boraston^^^
| Glycoside Hydrolase Family GH107 | |
| Clan | GH-R |
| Mechanism | retaining |
| Active site residues | known |
| CAZy DB link | |
| http://www.cazy.org/GH107.html | |
Substrate specificities
The glycoside hydrolases of this family are endo-acting α-fucosidases active on sulfated fucans (or fucoidans) from brown algae. All described GH107 family members are endo-1,4-fucanase of bacterial origin, and together with enzymes from the CAZY family GH29, they form the clan GH-R. Members of the GH107 family were first described in 2006.[1]
Kinetics and Mechanism
The mechanism was proven to be retaining by observation of the formation of an alpha-linked mercaptoethanol by transglycosylation.[2] It should then follow a classical Koshland double-displacement mechanism similarly to GH29.
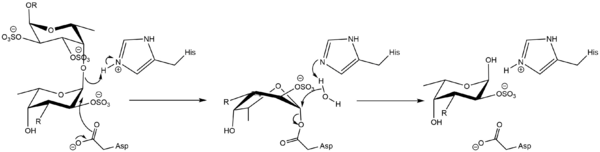
Catalytic Residues
The catalytic nucleophile is an aspartate, while the catalytic acid-base is a histidine. The later is unusual in GHs, and a divergence from GH29, but is likely necessary to avoid electronic repulsion with the substrate sulfate groups. These two residues have been identified by structural superimposition with GH29 enzymes, and are conserved within the few members of the GH107 family. The catalytic His has been confirmed by the lack of activity of th H294Q mutant of Mariniflexile fucanivorans, despite its structure was maintained.[2]
Three-dimensional structures
The crystal structures of Mariniflexile fucanivorans (PDB: 6dns,6dms,6dlh) and Psychromonas sp. (PDB: 6m8n) have been determined in 2018.[2] ThePsychromonas sp. (PDB: 6m8n) enzyme showed a single catalytic domain with a (β/α)8 / TIM-barrel fold, while in the Mariniflexile fucanivorans enzyme, this catalytic domain is followed by three Ig-like domains that wrap around the catalytic one.[2]
Family Firsts
- First stereochemistry determination
- Content is to be added here.
- First catalytic nucleophile identification
- Both catalytic residues have been identified at the same time, in 2018.[2]
- First general acid/base residue identification
- Both catalytic residues have been identified at the same time, in 2018.[2]
- First 3-D structure
- The crystal structures of Mariniflexile fucanivorans (PDB: 6dns,6dms,6dlh) and Psychromonas sp. (PDB: 6m8n) have been released at the same time, in 2018.[2]
References
- Colin S, Deniaud E, Jam M, Descamps V, Chevolot Y, Kervarec N, Yvin JC, Barbeyron T, Michel G, and Kloareg B. (2006). Cloning and biochemical characterization of the fucanase FcnA: definition of a novel glycoside hydrolase family specific for sulfated fucans. Glycobiology. 2006;16(11):1021-32. DOI:10.1093/glycob/cwl029 |
- Vickers C, Liu F, Abe K, Salama-Alber O, Jenkins M, Springate CMK, Burke JE, Withers SG, and Boraston AB. (2018). Endo-fucoidan hydrolases from glycoside hydrolase family 107 (GH107) display structural and mechanistic similarities to α-l-fucosidases from GH29. J Biol Chem. 2018;293(47):18296-18308. DOI:10.1074/jbc.RA118.005134 |
- Cantarel BL, Coutinho PM, Rancurel C, Bernard T, Lombard V, and Henrissat B. (2009). The Carbohydrate-Active EnZymes database (CAZy): an expert resource for Glycogenomics. Nucleic Acids Res. 2009;37(Database issue):D233-8. DOI:10.1093/nar/gkn663 |
-
Davies, G.J. and Sinnott, M.L. (2008) Sorting the diverse: the sequence-based classifications of carbohydrate-active enzymes. The Biochemist, vol. 30, no. 4., pp. 26-32. Download PDF version.