CAZypedia needs your help! We have many unassigned GH, PL, CE, AA, GT, and CBM pages in need of Authors and Responsible Curators.
Scientists at all career stages, including students, are welcome to contribute to CAZypedia. Read more here, and in the 10th anniversary article in Glycobiology.
New to the CAZy classification? Read this first.
*
Consider attending the 15th Carbohydrate Bioengineering Meeting in Ghent, 5-8 May 2024.
Glycoside Hydrolase Family 52
This page is currently under construction. This means that the Responsible Curator has deemed that the page's content is not quite up to CAZypedia's standards for full public consumption. All information should be considered to be under revision and may be subject to major changes.
- Author: ^^^Julie Grondin^^^
- Responsible Curator:
| Glycoside Hydrolase Family GH52 | |
| Clan | GH-O |
| Mechanism | retaining |
| Active site residues | known |
| CAZy DB link | |
| http://www.cazy.org/GH52.html | |
Substrate specificities
GH52 enzymes are bacterial exo-β-xylosidases (EC 3.2.1.37), which cleave xylose from the nonreducing end of xylooligosaccharides. Activity has been demonstrated on pNP-β-d-xylopyranoside [1, 2], xylobiose [2], xylotriose [2].
Kinetics and Mechanism
GH52 are retaining enzymes, proceeding via a classical Koshland double-displacement mechanism [3]. This was first shown by 1H-NMR in the cleavage of pNP-β-D-xylopyranoside by XynB2 from Bacillus stearothermophilus T-6 [1].
Catalytic Residues
Site-directed mutagenesis, chemical rescue, and kinetic profiling of XynB2 from Bacillus stearothermophilus T-6 identified E335 as the catalytic nucleophile, and D495 as the general acid/base [1, 4]. These results were further confirmed following the structural analysis of GH52 from Geobacillus thermoglucosidasius [2], their 6.5Å separation in the active site consistent with other retaining enzymes
Three-dimensional structures
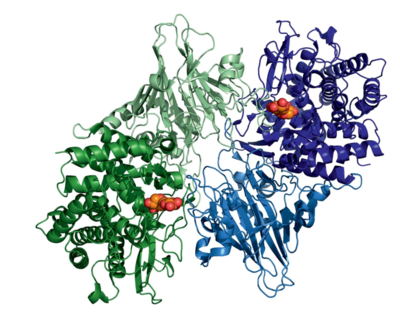
The structure of GH52 consists of an N-terminal β-sandwich domain and a C-terminal (a/a)6 barrel domain, classifying these enzymes into the GH-O clan.
The exo-acting mode of action of GH52 is reflected in the topology of the active site. The enzyme acts as a dimer in solution [1, 2], with interactions between monomers forming a deep pocket to enclose and distort the non-reducing end xylose into a high-energy 4H3 half-chair transition conformation, while simultaneously hindering the entry of large xylan polymers into the catalytic site [2].
Family Firsts
- First stereochemistry determination
- XynB2 from Bacillus stearothermophilus T-6 by 1H-NMR for the hydrolysis of pNP-β-D-xylopyranoside [1].
- First catalytic nucleophile identification
- XynB2 from Bacillus stearothermophilus T-6 by site-directed mutagenesis and chemical rescue [4].
- First general acid/base residue identification
- XynB2 from Bacillus stearothermophilus T-6 by site-directed mutagenesis, chemical rescue, and pH profiling [4].
- First 3-D structure
- GH52 from Geobacillus thermoglucosidasius NBRC 107763 [2].
References
- Bravman T, Zolotnitsky G, Shulami S, Belakhov V, Solomon D, Baasov T, Shoham G, and Shoham Y. (2001). Stereochemistry of family 52 glycosyl hydrolases: a beta-xylosidase from Bacillus stearothermophilus T-6 is a retaining enzyme. FEBS Lett. 2001;495(1-2):39-43. DOI:10.1016/s0014-5793(01)02360-2 |
- Espina G, Eley K, Pompidor G, Schneider TR, Crennell SJ, and Danson MJ. (2014). A novel β-xylosidase structure from Geobacillus thermoglucosidasius: the first crystal structure of a glycoside hydrolase family GH52 enzyme reveals unpredicted similarity to other glycoside hydrolase folds. Acta Crystallogr D Biol Crystallogr. 2014;70(Pt 5):1366-74. DOI:10.1107/S1399004714002788 |
-
Koshland DE Jr: Stereochemistry and the mechanism of enzyme reactions. Biol Rev 1953, 28:416-436. DOI:10.1111/j.1469-185X.1953.tb01386.x
- Bravman T, Belakhov V, Solomon D, Shoham G, Henrissat B, Baasov T, and Shoham Y. (2003). Identification of the catalytic residues in family 52 glycoside hydrolase, a beta-xylosidase from Geobacillus stearothermophilus T-6. J Biol Chem. 2003;278(29):26742-9. DOI:10.1074/jbc.M304144200 |