CAZypedia needs your help! We have many unassigned GH, PL, CE, AA, GT, and CBM pages in need of Authors and Responsible Curators.
Scientists at all career stages, including students, are welcome to contribute to CAZypedia. Read more here, and in the 10th anniversary article in Glycobiology.
New to the CAZy classification? Read this first.
*
Consider attending the 15th Carbohydrate Bioengineering Meeting in Ghent, 5-8 May 2024.
Difference between revisions of "Glycoside Hydrolase Family 58"
| Line 40: | Line 40: | ||
The structure for the K1F tailspike enzyme has been solved by Stummeyer et al. <cite>Stummeyer</cite>. The protein structure is similar to other phage tailspike endo-hydrolases in that it is a an extended molecule that is trimeric. The first tailspike enzyme to be studied in detail was the endo-rhamnosidase from the Salmonella phage P22 <cite>Steinbacher</cite>, which is in Family GH90. | The structure for the K1F tailspike enzyme has been solved by Stummeyer et al. <cite>Stummeyer</cite>. The protein structure is similar to other phage tailspike endo-hydrolases in that it is a an extended molecule that is trimeric. The first tailspike enzyme to be studied in detail was the endo-rhamnosidase from the Salmonella phage P22 <cite>Steinbacher</cite>, which is in Family GH90. | ||
| − | [[Image:Gh581.png|GH-58]] | + | [[Image:Gh581.png|GH-58]] [[Image:Gh58_2.png|GH-58]] |
== Family Firsts == | == Family Firsts == | ||
Revision as of 15:08, 13 January 2010
This page is currently under construction. This means that the Responsible Curator has deemed that the page's content is not quite up to CAZypedia's standards for full public consumption. All information should be considered to be under revision and may be subject to major changes.
- Author: ^^^Warren Wakarchuk^^^
- Responsible Curator: ^^^Warren Wakarchuk^^^
| Glycoside Hydrolase Family GH58 | |
| Clan | none |
| Mechanism | inverting |
| Active site residues | not known |
| CAZy DB link | |
| http://www.cazy.org/fam/GH58.html | |
Substrate specificities
Glycoside hydrolases of family 58 are endo-N-acetylneuraminidases (also termed endo-sialidases). The capsular coat surrounding E. coli K1, is poly-alpha-2,8-sialic acid and protects the bacterium from the host immune system, but it also acts as an anchor point for bacteriophage infection. A range of bacteriophages specific to this E. coli capsule type have been described which have tailspike enzymes that degrade the capuslar material [1, 2, 3]
Kinetics and Mechanism
Family 58 endo-sialidases are specific for polysialic acid and form a unique family of endo-acting sialidases, all others previously reported being exo-acting. The recently determined structure of an endo-sialidase derived from bacteriophage K1F (endoNF)[4] revealed the active site to lack a number of the residues that are conserved in other sialidases, implying a new, endo-sialidase-specific catalytic mechanism. Using synthetic trifluoromethylumbelliferyl oligosialoside substrates kinetic parameters for hydrolysis at a single cleavage site were determined. Measurement of kcat/Km at a series of pH values revealed a dependence on a single protonated group of pKa 5. Direct 1H-NMR analysis of the hydrolysis of trifluoromethylumbelliferyl sialotrioside revealed that endoNF undergoes catalysis with and inverting mechanism [5].
Catalytic Residues
From the crystal structure of the GH-58 enzyme it was noted that the normal catalyic residues found in exo-sialidases were not present. What was present were three conserved amino acids (Arg596,Arg647 and Glu581)that contribute to endosialidase activity. Single mutations of those residues reduced the level of activity, but not enough to mark the residues as absolutely required, but when double mutants were examined there was a complete loss of hydrolysis, but binding to PSA still occured [4]. Since this enzyme proceeds with inverting stereochemistry, a catalytic base is implied by the mechanism, and Glu581 might fill that role [5].
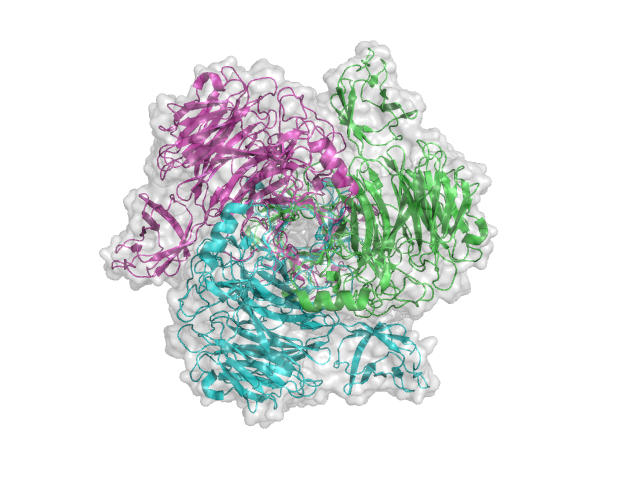
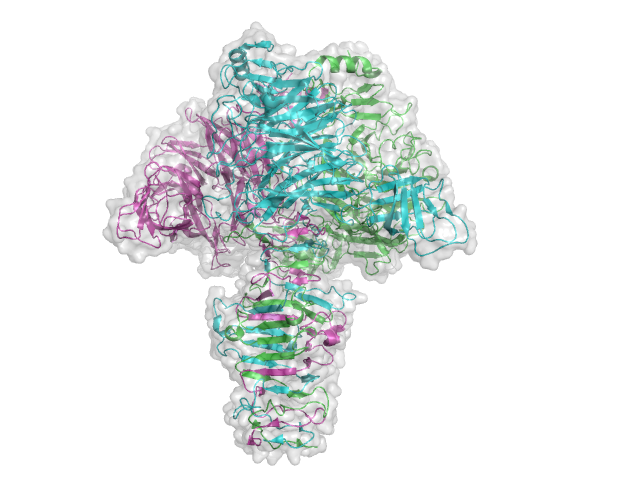
Three-dimensional structures
The structure for the K1F tailspike enzyme has been solved by Stummeyer et al. [4]. The protein structure is similar to other phage tailspike endo-hydrolases in that it is a an extended molecule that is trimeric. The first tailspike enzyme to be studied in detail was the endo-rhamnosidase from the Salmonella phage P22 [6], which is in Family GH90.
Family Firsts
- First sterochemistry determination
- The stereochemical outcome of this reaction was determined in the Withers laboratory at UBC using synthetic substrates with a trifluoromethyl-umbelliferol fluorophore [5]. This determination showed that unlike all other known sialidases, the endo-sialidase uses an inverting mechanism.
- First catalytic nucleophile identification
- Since this enzyme proceeds with inversion of sterechemistry, is does not use a catalytic nuceleophile.
- First general acid/base residue identification
It has been suggested though not conclusively proven that Glu581 is the catalytic base for this reaction [4], [5].
- First 3-D structure
- The first structure determination was performed with a truncated version of the K1F protein [4].
References
- Kwiatkowski B, Boschek B, Thiele H, and Stirm S. (1982). Endo-N-acetylneuraminidase associated with bacteriophage particles. J Virol. 1982;43(2):697-704. DOI:10.1128/JVI.43.2.697-704.1982 |
- Hallenbeck PC, Vimr ER, Yu F, Bassler B, and Troy FA. (1987). Purification and properties of a bacteriophage-induced endo-N-acetylneuraminidase specific for poly-alpha-2,8-sialosyl carbohydrate units. J Biol Chem. 1987;262(8):3553-61. | Google Books | Open Library
- Petter JG and Vimr ER. (1993). Complete nucleotide sequence of the bacteriophage K1F tail gene encoding endo-N-acylneuraminidase (endo-N) and comparison to an endo-N homolog in bacteriophage PK1E. J Bacteriol. 1993;175(14):4354-63. DOI:10.1128/jb.175.14.4354-4363.1993 |
- Stummeyer K, Dickmanns A, Mühlenhoff M, Gerardy-Schahn R, and Ficner R. (2005). Crystal structure of the polysialic acid-degrading endosialidase of bacteriophage K1F. Nat Struct Mol Biol. 2005;12(1):90-6. DOI:10.1038/nsmb874 |
- Morley TJ, Willis LM, Whitfield C, Wakarchuk WW, and Withers SG. (2009). A new sialidase mechanism: bacteriophage K1F endo-sialidase is an inverting glycosidase. J Biol Chem. 2009;284(26):17404-10. DOI:10.1074/jbc.M109.003970 |
- Steinbacher S, Seckler R, Miller S, Steipe B, Huber R, and Reinemer P. (1994). Crystal structure of P22 tailspike protein: interdigitated subunits in a thermostable trimer. Science. 1994;265(5170):383-6. DOI:10.1126/science.8023158 |