CAZypedia needs your help! We have many unassigned GH, PL, CE, AA, GT, and CBM pages in need of Authors and Responsible Curators.
Scientists at all career stages, including students, are welcome to contribute to CAZypedia. Read more here, and in the 10th anniversary article in Glycobiology.
New to the CAZy classification? Read this first.
*
Consider attending the 15th Carbohydrate Bioengineering Meeting in Ghent, 5-8 May 2024.
Difference between revisions of "Glycoside Hydrolase Family 73"
| Line 28: | Line 28: | ||
== Substrate specificities == | == Substrate specificities == | ||
| − | Family GH73 contains bacterial and prokaryotic viral [[glycoside hydrolase]]s. Most of the enzymes of this family are peptidoglycan hydrolases that cleave the β-1,4-glycosidic linkage between N-acetylglucosaminyl (NAG) and N-acetylmuramyl (NAM) moieties in the carbohydrate backbone of bacterial peptidoglycan. Because of their cleavage specificity, they are commonly described as N-acetylglucosaminidases. | + | Family GH73 contains bacterial and prokaryotic viral [[glycoside hydrolase]]s. Most of the enzymes of this family are peptidoglycan hydrolases that cleave the β-1,4-glycosidic linkage between N-acetylglucosaminyl (NAG) and N-acetylmuramyl (NAM) moieties in the carbohydrate backbone of bacterial peptidoglycan. Because of their cleavage specificity, they are commonly described as β-N-acetylglucosaminidases. |
| − | The | + | The enzymes from family GH73 are mainly involved in daughter cell separation during vegetative growth, and they often hydrolyse the septum after cell division (Acp from ''Clostridium perfringens'' <cite>Camiade2010</cite> AltA from ''Enterococcus faecalis'' <cite>Eckert2006</cite>). More rarely GH73 enzymes are used for host-cell invasion such as the virulence-associated peptidoglycan hydrolase Auto from ''Listeria monocytogene'' <cite>Bublitz2009</cite>. |
The GH73 are mostly surface located and exhibit repeated sequences that could be involved in cell-wall binding and therefore reinforce the enzymes catalytic activity. Unknown repeated domains are appended for instance to LytD and LytG from ''Bacillus subtilis'' <cite>Rashid1995 Horsburgh2003</cite>, AcmB from ''Lactococcus lactis'' <cite>Huard2003</cite> and Auto from ''L. monocytogene'' <cite>Bublitz2009</cite>. Some of these repeated domains have been identified like CBM50 also known as LysM domain appended to AcmA for Lactococcus lactis <cite>Inagaki2009</cite>, AltA from ''Enterococcus faecalis'' <cite>Eckert2006</cite> and Mur2-Mur2 from ''Enterococcus hirae'' <cite>Eckert2007</cite>. | The GH73 are mostly surface located and exhibit repeated sequences that could be involved in cell-wall binding and therefore reinforce the enzymes catalytic activity. Unknown repeated domains are appended for instance to LytD and LytG from ''Bacillus subtilis'' <cite>Rashid1995 Horsburgh2003</cite>, AcmB from ''Lactococcus lactis'' <cite>Huard2003</cite> and Auto from ''L. monocytogene'' <cite>Bublitz2009</cite>. Some of these repeated domains have been identified like CBM50 also known as LysM domain appended to AcmA for Lactococcus lactis <cite>Inagaki2009</cite>, AltA from ''Enterococcus faecalis'' <cite>Eckert2006</cite> and Mur2-Mur2 from ''Enterococcus hirae'' <cite>Eckert2007</cite>. | ||
Revision as of 07:42, 2 September 2010
This page is currently under construction. This means that the Responsible Curator has deemed that the page's content is not quite up to CAZypedia's standards for full public consumption. All information should be considered to be under revision and may be subject to major changes.
- Author: ^^^Florence Vincent^^^
- Responsible Curator: ^^^Bernard Henrissat^^^
| Glycoside Hydrolase Family GH73 | |
| Clan | none, α+β "lysozyme fold" |
| Mechanism | not known |
| Active site residues | partially known |
| CAZy DB link | |
| http://www.cazy.org/GH73.html | |
Substrate specificities
Family GH73 contains bacterial and prokaryotic viral glycoside hydrolases. Most of the enzymes of this family are peptidoglycan hydrolases that cleave the β-1,4-glycosidic linkage between N-acetylglucosaminyl (NAG) and N-acetylmuramyl (NAM) moieties in the carbohydrate backbone of bacterial peptidoglycan. Because of their cleavage specificity, they are commonly described as β-N-acetylglucosaminidases. The enzymes from family GH73 are mainly involved in daughter cell separation during vegetative growth, and they often hydrolyse the septum after cell division (Acp from Clostridium perfringens [1] AltA from Enterococcus faecalis [2]). More rarely GH73 enzymes are used for host-cell invasion such as the virulence-associated peptidoglycan hydrolase Auto from Listeria monocytogene [3]. The GH73 are mostly surface located and exhibit repeated sequences that could be involved in cell-wall binding and therefore reinforce the enzymes catalytic activity. Unknown repeated domains are appended for instance to LytD and LytG from Bacillus subtilis [4, 5], AcmB from Lactococcus lactis [6] and Auto from L. monocytogene [3]. Some of these repeated domains have been identified like CBM50 also known as LysM domain appended to AcmA for Lactococcus lactis [7], AltA from Enterococcus faecalis [2] and Mur2-Mur2 from Enterococcus hirae [8].
Kinetics and Mechanism
No kinetic parameters have been determined for any enzyme of the GH73 family, as the production of synthetic peptidoglycan substrates remains a challenging task.
Catalytic Residues
The catalytic general acid is a glutamate, strictly conserved in the GH73 family. Its catalytic role has been evidenced in FlgJ [9], Auto [3], AcmA [7] and AltWN [10]. Glu185 in FlgJ and Glu122 in Auto have also been identified through structural comparison with the actives sites from GH23, GH22 and GH19 enzymes [3, 11]. Nevertheless, both structures of FlgJ and Auto have in common the evident lack of a nearby second catalytic carboxylate, provided for instance by Asp52(53) in GH22 lysozymes (see figure 1). In FlgJ and Auto the catalytic nucleophile/general base, a Glu corresponding to Aps52, is strickly conserved in the GH73 family but is situated 13Å away from the Glu general acid in the active site.
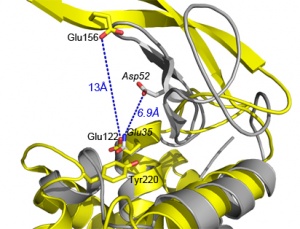
Mutational analysis on the putative distant nucleophile (Glu156) in Auto, showed a drastic decrease of the catalytic activity [3]. Therefore, Bublitz et al proposed a single displacement mechanism involving a distant carboxylate that would serve as a base assisting a water molecule for the nucleophilic attack on the opposite side of the sugar ring (inverting mechanism). This mechanism also involves an important displacement on the β-lobe upon substrat binding that would bring the nucleophile/base closer to the active site.
On the other hand, mutational analyses on FlgJ , AcmA and AltWN revealed an uncertainty on the nucleophile/base residue and the putative existence of another key catalytic residue. It is noteworthy that only the mutational analysis on Auto revealed a decreased catalytic activity when the nucleophile Glu156 was mutated into glutamine. In FlgJ, AcmA and AltWN, an important residual activity upon mutation of this equivalent Glu into alanine, glutamine or asparagine (for Asp1275 in AltWN) ruled out this residue as a key catalytic residue.
In close proximity to the Glu proton donor is a Tyrosine highly conserved in the GH73 family (Fig1: Tyr220 in Auto). Amino acid substitution of this tyrosine on FlgJ, AcmA and AltWN exhibited reduced activity similar to the mutation of the Glu proton donor [7, 9, 10]. The substitution of this Tyr into a Phe or Trp, in AcmA and AltWN, retained substantial activity.
Inagaki and Murayama agreed on the fact that the Glu proton donor and this nearby Tyr are probably crucial for enzyme activities of FlgJ, AcmA, and AltWN. The role of the Tyr have already been discussed for Auto, they suggested the need for an hydrophobic residue in this position, to protonate the carboxylate group of the proton donor and maintain the stable conformation of the active site residues [3].
Finally, based on sequence analyses in the GH73 family and in comparison with families GH20, GH18, GH23 and GH56, enzymes that do not have a catalytic nucleophile residue, Inagaki et al suggested a neighboring group participation involving the Glu proton donor and the Tyr as essential catalytic residues. This mechanism implies that the 2-acetamido group of the NAG is acting as an intramolecular nucleophile [7].
Three-dimensional structures
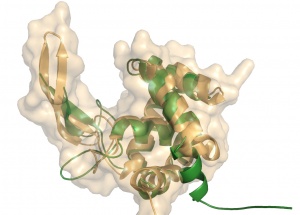
Crystal structures of GH73 are available and have been coincidently reported, FlgJ from Sphingomonas sp. (SPH1045-C) [11] and Auto a virulence associated peptigoglycan hydrolase from Listeria monocytogenes [3]. A structure for a catalytic mutant (E185A) of FlgJ has been solved by Maruyama et al [9] but doesn’t show any conformational changes. The two GH73 show the same fold, with two subdomains consisting of a β-lobe and an α-lobe that together create an extended substrate binding groove (Figure 2). With a typical lysozyme (α+β) fold, the catalytic domain of Auto is structurally related to the catalytic domain of Slt70 from E. coli [12], the family GH19 chitinases and goose egg-white lysozyme (GEWL, GH23)[13]. FlgJ is structurally related to a peptidoglycan degrading enzyme from the bacteriophage phi 29 [14] and also to family GH22 and GH23 lysozymes.
Family Firsts
- First stereochemistry determination
- First catalytic nucleophile identification
- Evidence for a putative nucleophile residue in Auto, a peptidoglycan hydrolase from Lytseria monocytogene [3]
- First general acid/base/general acid residue identification
- First 3-D structure
- peptidoglycan hydrolase FlgJ from Sphingomonas sp. [11]
References
- Camiade E, Peltier J, Bourgeois I, Couture-Tosi E, Courtin P, Antunes A, Chapot-Chartier MP, Dupuy B, and Pons JL. (2010). Characterization of Acp, a peptidoglycan hydrolase of Clostridium perfringens with N-acetylglucosaminidase activity that is implicated in cell separation and stress-induced autolysis. J Bacteriol. 2010;192(9):2373-84. DOI:10.1128/JB.01546-09 |
- Eckert C, Lecerf M, Dubost L, Arthur M, and Mesnage S. (2006). Functional analysis of AtlA, the major N-acetylglucosaminidase of Enterococcus faecalis. J Bacteriol. 2006;188(24):8513-9. DOI:10.1128/JB.01145-06 |
- Bublitz M, Polle L, Holland C, Heinz DW, Nimtz M, and Schubert WD. (2009). Structural basis for autoinhibition and activation of Auto, a virulence-associated peptidoglycan hydrolase of Listeria monocytogenes. Mol Microbiol. 2009;71(6):1509-22. DOI:10.1111/j.1365-2958.2009.06619.x |
- Rashid MH, Mori M, and Sekiguchi J. (1995). Glucosaminidase of Bacillus subtilis: cloning, regulation, primary structure and biochemical characterization. Microbiology (Reading). 1995;141 ( Pt 10):2391-404. DOI:10.1099/13500872-141-10-2391 |
- Horsburgh GJ, Atrih A, Williamson MP, and Foster SJ. (2003). LytG of Bacillus subtilis is a novel peptidoglycan hydrolase: the major active glucosaminidase. Biochemistry. 2003;42(2):257-64. DOI:10.1021/bi020498c |
- Huard C, Miranda G, Wessner F, Bolotin A, Hansen J, Foster SJ, and Chapot-Chartier MP. (2003). Characterization of AcmB, an N-acetylglucosaminidase autolysin from Lactococcus lactis. Microbiology (Reading). 2003;149(Pt 3):695-705. DOI:10.1099/mic.0.25875-0 |
- Inagaki N, Iguchi A, Yokoyama T, Yokoi KJ, Ono Y, Yamakawa A, Taketo A, and Kodaira K. (2009). Molecular properties of the glucosaminidase AcmA from Lactococcus lactis MG1363: mutational and biochemical analyses. Gene. 2009;447(2):61-71. DOI:10.1016/j.gene.2009.08.004 |
- Eckert C, Magnet S, and Mesnage S. (2007). The Enterococcus hirae Mur-2 enzyme displays N-acetylglucosaminidase activity. FEBS Lett. 2007;581(4):693-6. DOI:10.1016/j.febslet.2007.01.033 |
- Maruyama Y, Ochiai A, Itoh T, Mikami B, Hashimoto W, and Murata K. (2010). Mutational studies of the peptidoglycan hydrolase FlgJ of Sphingomonas sp. strain A1. J Basic Microbiol. 2010;50(4):311-7. DOI:10.1002/jobm.200900249 |
- Yokoi KJ, Sugahara K, Iguchi A, Nishitani G, Ikeda M, Shimada T, Inagaki N, Yamakawa A, Taketo A, and Kodaira K. (2008). Molecular properties of the putative autolysin Atl(WM) encoded by Staphylococcus warneri M: mutational and biochemical analyses of the amidase and glucosaminidase domains. Gene. 2008;416(1-2):66-76. DOI:10.1016/j.gene.2008.03.004 |
- Hashimoto W, Ochiai A, Momma K, Itoh T, Mikami B, Maruyama Y, and Murata K. (2009). Crystal structure of the glycosidase family 73 peptidoglycan hydrolase FlgJ. Biochem Biophys Res Commun. 2009;381(1):16-21. DOI:10.1016/j.bbrc.2009.01.186 |
- van Asselt EJ, Thunnissen AM, and Dijkstra BW. (1999). High resolution crystal structures of the Escherichia coli lytic transglycosylase Slt70 and its complex with a peptidoglycan fragment. J Mol Biol. 1999;291(4):877-98. DOI:10.1006/jmbi.1999.3013 |
- Weaver LH, Grütter MG, and Matthews BW. (1995). The refined structures of goose lysozyme and its complex with a bound trisaccharide show that the "goose-type" lysozymes lack a catalytic aspartate residue. J Mol Biol. 1995;245(1):54-68. DOI:10.1016/s0022-2836(95)80038-7 |
- Xiang Y, Morais MC, Cohen DN, Bowman VD, Anderson DL, and Rossmann MG. (2008). Crystal and cryoEM structural studies of a cell wall degrading enzyme in the bacteriophage phi29 tail. Proc Natl Acad Sci U S A. 2008;105(28):9552-7. DOI:10.1073/pnas.0803787105 |