CAZypedia needs your help! We have many unassigned GH, PL, CE, AA, GT, and CBM pages in need of Authors and Responsible Curators.
Scientists at all career stages, including students, are welcome to contribute to CAZypedia. Read more here, and in the 10th anniversary article in Glycobiology.
New to the CAZy classification? Read this first.
*
Consider attending the 15th Carbohydrate Bioengineering Meeting in Ghent, 5-8 May 2024.
Difference between revisions of "Glycoside Hydrolase Family 99"
m (Reverted edits by Spencer Williams (talk) to last revision by Harry Brumer) |
|||
| (42 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
| − | + | {{CuratorApproved}} | |
| − | |||
* [[Author]]: ^^^Spencer Williams^^^ | * [[Author]]: ^^^Spencer Williams^^^ | ||
* [[Responsible Curator]]: ^^^Gideon Davies^^^ | * [[Responsible Curator]]: ^^^Gideon Davies^^^ | ||
---- | ---- | ||
| − | + | ||
<div style="float:right"> | <div style="float:right"> | ||
{| {{Prettytable}} | {| {{Prettytable}} | ||
| Line 12: | Line 11: | ||
|- | |- | ||
|'''Clan''' | |'''Clan''' | ||
| − | | | + | |not assigned |
|- | |- | ||
|'''Mechanism''' | |'''Mechanism''' | ||
| − | |retaining | + | |retaining |
|- | |- | ||
|'''Active site residues''' | |'''Active site residues''' | ||
| − | | | + | |known |
|- | |- | ||
|{{Hl2}} colspan="2" align="center" |'''CAZy DB link''' | |{{Hl2}} colspan="2" align="center" |'''CAZy DB link''' | ||
| Line 27: | Line 26: | ||
<!-- This is the end of the table --> | <!-- This is the end of the table --> | ||
| + | == Substrate specificities == | ||
| + | [[Image:GH99_substrates.png|thumb|right|300px|'''Figure 1. (Top) Endo-α-mannosidase is located in the Golgi apparatus and pre-Golgi intermediates and acts on folded and unfolded ER-escaped glucosylated N-linked glycoproteins (X = protein); glucosylated free oligosaccharides (X = OH); and dolichol-bound glucosylated glycans (X = diphosphodolichol). (Bottom) Endo-α-1,2-mannanases are bacterial enzymes with the ability to cleave Manα1-3Manα1-2Manα1-2Manα substructures within fungal cell wall mannan at the indicated positions, releasing α-1,3-mannobiose.''']] | ||
| + | [[Glycoside hydrolases]] of family GH99 are [[endo]]-acting enzymes. This family was originally created from the mammalian enzyme, cloned by Spiro and co-workers <cite>Spiro1997</cite>. Two main activities have been described for this family. Endo-α-1,2-mannosidase activity describes the capacity to cleave glucose-substituted mannose within immature N-linked glycans of the general formula Glc<sub>1-3</sub>Man<sub>9</sub>GlcNAc<sub>2</sub> (or structures trimmed in the B and C branches). On the other hand endo-α-1,2-mannanase activity describes the ability to cleave αMan-1,3-αMan-1,2-αMan-1,2-αMan sequences within fungal cell wall mannans, releasing α-1,3-mannobiose <cite>#Hakki2014</cite>. | ||
| − | + | Mammalian endo-α-mannosidases typically possess maximal activity on the monoglucosylated forms <cite>Roth2003</cite>. Mammalian endo-α-mannosidases are localized to the Golgi apparatus <cite>Zuber2000</cite> and appear to play a role in the rescue of glucosylated N-linked glycans that have evaded the action of the endoplasmic reticulum ''exo''-glucosidases I and II <cite>Dale1986</cite>. Mammalian [[endo]]-α-mannosidase has greater activity on glucosylated N-linked glycans that have been trimmed in the non-glucose-substituted branches <cite>Spiro1997</cite>. There is evidence that mammalian [[endo]]-α-mannosidases act on dolichol-bound N-glycan precursors <cite>Spiro2000</cite>, as well as free oligosaccharides released from N-glycoproteins and which undergo retrograde transport through the secretory pathway <cite>Kukushkin2011</cite>. A fluorescent tetrasaccharide fragment (Glcα1-3Manα1-2Manα1-2Manα1-O-C3H6-NH-Dansyl) has been developed that is a substrate for mammalian endo-α-mannosidase <cite>Iwamoto2014</cite>. Studies of bacterial GH99 enzymes from ''Shewanella amazonensis'' <cite>Matsuda2011</cite>, ''Bacteroides thetaiotaomicron'' and ''Bacteroides xylanisolvens'' <cite>Thompson2012</cite> have shown that these enzymes can also process Glc<sub>1/3</sub>Man<sub>9/7</sub>GlcNAc<sub>2</sub> structures, matching the substrate specificity of the mammalian enzymes. Kinetic analysis of ''Bacteroides thetaiotaomicron'' GH99 on a fluorescently-labelled Glc<sub>3</sub>Man<sub>9</sub>GlcNAc<sub>2</sub> structure yielded kinetic parameters that were similar to that found for the mammalian enzyme <cite>Thompson2012</cite>. Both mammalian and bacterial enzymes can utilize simple 'reducing end' substrate mimics such as methylumbelliferyl α-glucosyl-1,3-α-mannopyranoside <cite>Vogel2000</cite> or α-glucosyl-1,3-α-mannopyranosyl fluoride <cite>Thompson2012</cite>, but are inactive on aryl mannosides. | |
| − | + | ||
| + | Bacterial GH99 enzymes from ''B. thetaiotaomicron'' and ''B. xylanisolvens'' have superior kinetics for cleavage of Manα1-3Manα1-2Manα1-2Manα-OMe versus glucosylated high mannose N-glycans and are thus more appropriately considered endo-α-1,2-mannanases <cite>#Hakki2014</cite>. These two enzymes displayed a 10-fold preference for methylumbelliferyl α-mannosyl-1,3-α-mannopyranoside versus methylumbelliferyl α-glucosyl-1,3-α-mannopyranoside <cite>#Hakki2014</cite>. | ||
== Kinetics and Mechanism == | == Kinetics and Mechanism == | ||
| − | + | Family GH99 [[endo]]-α-mannosidases are [[retaining]] enzymes, as first shown by <sup>1</sup>H NMR analysis of the hydrolysis of α-glucosyl-1,3-α-mannopyranosyl fluoride by ''Bacteroides thetaiotaomicron'' <cite>Thompson2012</cite>. [[Retaining]] mannoside hydrolases (eg [[GH38]]) typically follow a [[classical Koshland double-displacement mechanism]]. However, X-ray crystallographic analysis of ''Bx''GH99 and ''Bt''GH99 failed to reveal a candidate nucleophilic residue; instead an alternative mechanism involving substrate-assisted catalysis by the 2OH residue and proceeding through a 1,2-anhydro sugar was proposed <cite>Thompson2012</cite>. In this proposal, Glu333 in ''BxGH99'' (Glu329 in ''Bt''GH99) acts as a [[general acid/base]] to deprotonate the 2OH and protonate the 1,2-anhydrosugar. | |
| + | |||
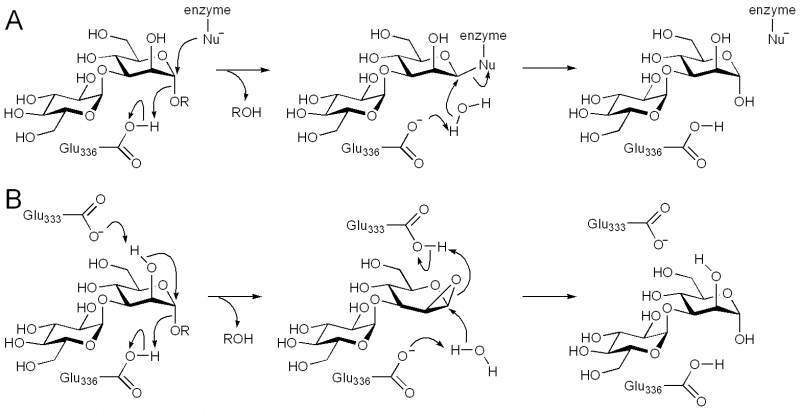
| + | [[Image:GH99_mechanisms.png|thumb|center|800px|'''Figure 2. Proposed catalytic mechanisms for GH99 endo-α-mannosidase. (A) [[Classical Koshland double-displacement mechanism]]. (B) Neighboring group participation via a 1,2-anhydrosugar intermediate.''']] | ||
== Catalytic Residues == | == Catalytic Residues == | ||
| − | + | The [[general acid/base]] was highlighted by X-ray crystallographic analysis as Glu336 in ''Bx''GH99 and Glu332 in ''Bt''GH99 <cite>Thompson2012</cite>. The Glu332Ala mutant of ''Bt''GH99 shows a 50-fold decrease in catalytic activity relative to the wild-type enzyme using the activated substrate α-glucosyl-1,3-α-mannopyranosyl fluoride, and undetectable activity against the natural substrate, Glc<sub>3</sub>Man<sub>7</sub>GlcNAc<sub>2</sub>, supporting the role of this residue as [[general acid/base]]. As described in "Kinetics and Mechanism" the identity of the nucleophilic residue used for catalysis is more obscure and the 2OH of the substrate has been proposed to act as a nucleophile in a mechanism involving substrate assisted catalysis <cite>Thompson2012</cite>. According to this proposal, Glu333 in ''Bx''GH99 (Glu329 in ''Bt''GH99) acts as a [[general acid/base]] to deprotonate the 2OH and protonate the 1,2-anhydrosugar. | |
== Three-dimensional structures == | == Three-dimensional structures == | ||
| − | + | Three-dimensional structures are available for bacterial members of GH99, including ''B. thetaiotaomicron'' and ''B. xylanisolvens'' <cite>Thompson2012</cite>. They have a classical (β/α)<sub>8</sub> TIM barrel fold, which is distinguished by the presence of extended loop motifs that form the active site. In different structures of the bacterial enzymes, these loops adopt different conformations, and appear to play a role in recognizing the extended structure of the N-glycan substrate. Binary complexes with two inhibitors, α-glucosyl-1,3-deoxymannonojirimycin and α-glucosyl-1,3-isofagomine, and 'active-site-spanning' ternary complexes with the same two inhibitors and the reducing end product fragment 1,2-α-mannobiose, provided insight into active site residues, especially the acid/base (Glu336 in ''Bx''GH99; Glu332 in ''Bt''GH99) and another key residue that interacts with both the 2OH of the -1 mannose residue and the 6OH of the -2 glucose residue, which provides a rationale for the requirement of a glucosylated-mannoside as the minimal substrate for GH99 enzymes <cite>Thompson2012</cite>. As discussed in more detail in the "Kinetics and Mechanism" section, the precise identity of the nucleophilic residue is unclear, as in all GH99-inhibitor complexes with an occupied -1 subsite there is no candidate nucleophile within a reasonable distance to the "anomeric" carbon: in ''Bx''GH99 Glu333 is approximately 3.5 Å distant and the OH of Tyr46 and Tyr252 are 4.0 Å distant. | |
| + | |||
| + | Comparison of binary complexes of α-mannosyl-1,3-isofagomine and α-glucosyl-1,3-isofagomine with ''B. xylanisolvens'' GH99 revealed almost identical binding <cite>#Hakki2014</cite>. It was speculated that a key residue in the -2 subsite, tryptophan-126 in ''Bx''GH99, provided a preference for mannose-configured substrates in these bacterial endo-α-1,2-mannanases. By contrast the equivalent residue in mammalian endo-α-mannosidases is a highly conserved tyrosine, which it was speculated may result in a favorable hydrogen bond to a glucose-configured substrate and confer endo-α-mannosidase activity. | ||
| + | |||
| + | A GH99-like protein from the marine flavobacterium ''Ochrovirga pacifica'', which lacks the conserved catalytic residues of glycoside hydrolase family 99 possessed a similar fold to other GH99 members. It is speculated that this protein may be noncatalytic and possibly involved in carbohydrate binding <cite>Robb2017</cite>. | ||
| + | |||
| + | <!-- This section has been inactivated owing to to browser-based problems running applets | ||
| + | |||
| + | === Sample structures === | ||
| + | |||
| + | {| {{Prettytable}} | ||
| + | |- | ||
| + | |||
| + | !style="width:50%"|Three-dimensional structure of GH99 endo-α-mannosidase from ''Bacteroides xylanisolvens'', PDB code [{{PDBlink}}4ad1] <cite>Thompson2012</cite>. | ||
| + | !style="width:50%"|Three-dimensional structure of GH99 endo-α-mannosidase from ''Bacteroides xylanisolvens'' bound to glucose-1,3-isofagomine and α-1,2- mannobiose, PDB code [{{PDBlink}}4ad4] <cite>Thompson2012</cite>. | ||
| + | |- | ||
| + | | | ||
| + | <jmol> | ||
| + | <jmolApplet> | ||
| + | <color>white</color> | ||
| + | <frame>true</frame> | ||
| + | <uploadedFileContents>4ad1.pdb</uploadedFileContents> | ||
| + | <script>cpk off; wireframe off; cartoon; color cartoon powderblue; select ligand; wireframe 0.3; select MG; spacefill; set spin Y 10; spin off; set antialiasDisplay OFF</script> | ||
| + | </jmolApplet> | ||
| + | </jmol> | ||
| + | |||
| + | | | ||
| + | <jmol> | ||
| + | <jmolApplet> | ||
| + | <color>white</color> | ||
| + | <frame>true</frame> | ||
| + | <uploadedFileContents>4ad4.pdb</uploadedFileContents> | ||
| + | <script>cpk off; wireframe off; cartoon; color cartoon powderblue; select GLC or IFG; wireframe 0.3; select MAN; wireframe 0.3; set spin Y 10; spin off; set antialiasDisplay OFF</script> | ||
| + | </jmolApplet> | ||
| + | </jmol> | ||
| + | |- | ||
| + | |} | ||
| + | <br style="clear: both" /> --> | ||
== Family Firsts == | == Family Firsts == | ||
| − | ;First | + | ;First sterochemistry determination: ''Bacteroides thetaiotaomicron'' ''endo''-α-mannosidase by <sup>1</sup>H NMR <cite>Thompson2012</cite> |
| − | ;First catalytic nucleophile identification: | + | |
| − | ;First general acid/base residue identification: | + | ;First [[catalytic nucleophile]] identification: It has been proposed that GH99 enzymes operate through a mechanism involving substrate assisted catalysis by the 2OH group of the -1 mannose residue <cite>Thompson2012</cite> |
| − | ;First 3-D structure: | + | |
| + | ;First [[general acid/base]] residue identification: ''Bacteroides thetaiotaomicron'' ''endo''-α-mannosidase by X-ray crystallography and analysis of enzyme mutant activities <cite>Thompson2012</cite> | ||
| + | |||
| + | ;First 3-D structure of a GH99 enzyme: ''Bacteroides thetaiotaomicron'' and ''Bacteroides xylanisolvens'' ''endo''-α-mannosidases <cite>Thompson2012</cite> | ||
== References == | == References == | ||
<biblio> | <biblio> | ||
| − | # | + | #Roth2003 pmid=12770767 |
| − | # | + | #Spiro1997 pmid=9361017 |
| + | #Zuber2000 pmid=11102520 | ||
| + | #Dale1986 pmid=3087421 | ||
| + | #Kukushkin2011 pmid=21585340 | ||
| + | #Spiro2000 pmid=10764841 | ||
| + | #Thompson2012 pmid=22219371 | ||
| + | #Matsuda2011 pmid=21512220 | ||
| + | #Vogel2000 Vogel C, Pohlentz G. ''Synthesis of α-D-glucopyranosyl-(1,3)-α-D-mannopyranosyl-(1,7)-4-methylumbelliferone, a fluorogenic substrate for endo-α-1,2-mannosidase.'' J. Carbohydr. Chem. 2000, 19: 1247-1258. | ||
| + | #Iwamoto2014 pmid=25036115 | ||
| + | #Hakki2014 pmid=25487964 | ||
</biblio> | </biblio> | ||
[[Category:Glycoside Hydrolase Families|GH099]] | [[Category:Glycoside Hydrolase Families|GH099]] | ||
Revision as of 04:22, 13 March 2018
This page has been approved by the Responsible Curator as essentially complete. CAZypedia is a living document, so further improvement of this page is still possible. If you would like to suggest an addition or correction, please contact the page's Responsible Curator directly by e-mail.
- Author: ^^^Spencer Williams^^^
- Responsible Curator: ^^^Gideon Davies^^^
| Glycoside Hydrolase Family GH99 | |
| Clan | not assigned |
| Mechanism | retaining |
| Active site residues | known |
| CAZy DB link | |
| http://www.cazy.org/GH99.html | |
Substrate specificities
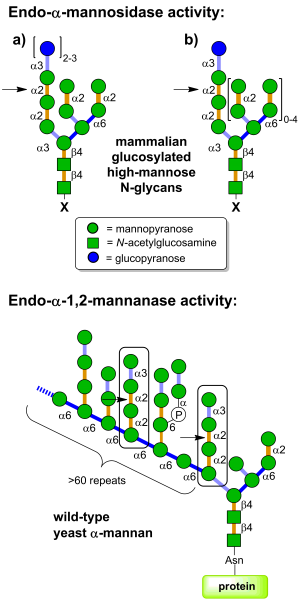
Glycoside hydrolases of family GH99 are endo-acting enzymes. This family was originally created from the mammalian enzyme, cloned by Spiro and co-workers [1]. Two main activities have been described for this family. Endo-α-1,2-mannosidase activity describes the capacity to cleave glucose-substituted mannose within immature N-linked glycans of the general formula Glc1-3Man9GlcNAc2 (or structures trimmed in the B and C branches). On the other hand endo-α-1,2-mannanase activity describes the ability to cleave αMan-1,3-αMan-1,2-αMan-1,2-αMan sequences within fungal cell wall mannans, releasing α-1,3-mannobiose [2].
Mammalian endo-α-mannosidases typically possess maximal activity on the monoglucosylated forms [3]. Mammalian endo-α-mannosidases are localized to the Golgi apparatus [4] and appear to play a role in the rescue of glucosylated N-linked glycans that have evaded the action of the endoplasmic reticulum exo-glucosidases I and II [5]. Mammalian endo-α-mannosidase has greater activity on glucosylated N-linked glycans that have been trimmed in the non-glucose-substituted branches [1]. There is evidence that mammalian endo-α-mannosidases act on dolichol-bound N-glycan precursors [6], as well as free oligosaccharides released from N-glycoproteins and which undergo retrograde transport through the secretory pathway [7]. A fluorescent tetrasaccharide fragment (Glcα1-3Manα1-2Manα1-2Manα1-O-C3H6-NH-Dansyl) has been developed that is a substrate for mammalian endo-α-mannosidase [8]. Studies of bacterial GH99 enzymes from Shewanella amazonensis [9], Bacteroides thetaiotaomicron and Bacteroides xylanisolvens [10] have shown that these enzymes can also process Glc1/3Man9/7GlcNAc2 structures, matching the substrate specificity of the mammalian enzymes. Kinetic analysis of Bacteroides thetaiotaomicron GH99 on a fluorescently-labelled Glc3Man9GlcNAc2 structure yielded kinetic parameters that were similar to that found for the mammalian enzyme [10]. Both mammalian and bacterial enzymes can utilize simple 'reducing end' substrate mimics such as methylumbelliferyl α-glucosyl-1,3-α-mannopyranoside [11] or α-glucosyl-1,3-α-mannopyranosyl fluoride [10], but are inactive on aryl mannosides.
Bacterial GH99 enzymes from B. thetaiotaomicron and B. xylanisolvens have superior kinetics for cleavage of Manα1-3Manα1-2Manα1-2Manα-OMe versus glucosylated high mannose N-glycans and are thus more appropriately considered endo-α-1,2-mannanases [2]. These two enzymes displayed a 10-fold preference for methylumbelliferyl α-mannosyl-1,3-α-mannopyranoside versus methylumbelliferyl α-glucosyl-1,3-α-mannopyranoside [2].
Kinetics and Mechanism
Family GH99 endo-α-mannosidases are retaining enzymes, as first shown by 1H NMR analysis of the hydrolysis of α-glucosyl-1,3-α-mannopyranosyl fluoride by Bacteroides thetaiotaomicron [10]. Retaining mannoside hydrolases (eg GH38) typically follow a classical Koshland double-displacement mechanism. However, X-ray crystallographic analysis of BxGH99 and BtGH99 failed to reveal a candidate nucleophilic residue; instead an alternative mechanism involving substrate-assisted catalysis by the 2OH residue and proceeding through a 1,2-anhydro sugar was proposed [10]. In this proposal, Glu333 in BxGH99 (Glu329 in BtGH99) acts as a general acid/base to deprotonate the 2OH and protonate the 1,2-anhydrosugar.
Catalytic Residues
The general acid/base was highlighted by X-ray crystallographic analysis as Glu336 in BxGH99 and Glu332 in BtGH99 [10]. The Glu332Ala mutant of BtGH99 shows a 50-fold decrease in catalytic activity relative to the wild-type enzyme using the activated substrate α-glucosyl-1,3-α-mannopyranosyl fluoride, and undetectable activity against the natural substrate, Glc3Man7GlcNAc2, supporting the role of this residue as general acid/base. As described in "Kinetics and Mechanism" the identity of the nucleophilic residue used for catalysis is more obscure and the 2OH of the substrate has been proposed to act as a nucleophile in a mechanism involving substrate assisted catalysis [10]. According to this proposal, Glu333 in BxGH99 (Glu329 in BtGH99) acts as a general acid/base to deprotonate the 2OH and protonate the 1,2-anhydrosugar.
Three-dimensional structures
Three-dimensional structures are available for bacterial members of GH99, including B. thetaiotaomicron and B. xylanisolvens [10]. They have a classical (β/α)8 TIM barrel fold, which is distinguished by the presence of extended loop motifs that form the active site. In different structures of the bacterial enzymes, these loops adopt different conformations, and appear to play a role in recognizing the extended structure of the N-glycan substrate. Binary complexes with two inhibitors, α-glucosyl-1,3-deoxymannonojirimycin and α-glucosyl-1,3-isofagomine, and 'active-site-spanning' ternary complexes with the same two inhibitors and the reducing end product fragment 1,2-α-mannobiose, provided insight into active site residues, especially the acid/base (Glu336 in BxGH99; Glu332 in BtGH99) and another key residue that interacts with both the 2OH of the -1 mannose residue and the 6OH of the -2 glucose residue, which provides a rationale for the requirement of a glucosylated-mannoside as the minimal substrate for GH99 enzymes [10]. As discussed in more detail in the "Kinetics and Mechanism" section, the precise identity of the nucleophilic residue is unclear, as in all GH99-inhibitor complexes with an occupied -1 subsite there is no candidate nucleophile within a reasonable distance to the "anomeric" carbon: in BxGH99 Glu333 is approximately 3.5 Å distant and the OH of Tyr46 and Tyr252 are 4.0 Å distant.
Comparison of binary complexes of α-mannosyl-1,3-isofagomine and α-glucosyl-1,3-isofagomine with B. xylanisolvens GH99 revealed almost identical binding [2]. It was speculated that a key residue in the -2 subsite, tryptophan-126 in BxGH99, provided a preference for mannose-configured substrates in these bacterial endo-α-1,2-mannanases. By contrast the equivalent residue in mammalian endo-α-mannosidases is a highly conserved tyrosine, which it was speculated may result in a favorable hydrogen bond to a glucose-configured substrate and confer endo-α-mannosidase activity.
A GH99-like protein from the marine flavobacterium Ochrovirga pacifica, which lacks the conserved catalytic residues of glycoside hydrolase family 99 possessed a similar fold to other GH99 members. It is speculated that this protein may be noncatalytic and possibly involved in carbohydrate binding [12].
Family Firsts
- First sterochemistry determination
- Bacteroides thetaiotaomicron endo-α-mannosidase by 1H NMR [10]
- First catalytic nucleophile identification
- It has been proposed that GH99 enzymes operate through a mechanism involving substrate assisted catalysis by the 2OH group of the -1 mannose residue [10]
- First general acid/base residue identification
- Bacteroides thetaiotaomicron endo-α-mannosidase by X-ray crystallography and analysis of enzyme mutant activities [10]
- First 3-D structure of a GH99 enzyme
- Bacteroides thetaiotaomicron and Bacteroides xylanisolvens endo-α-mannosidases [10]
References
- Spiro MJ, Bhoyroo VD, and Spiro RG. (1997). Molecular cloning and expression of rat liver endo-alpha-mannosidase, an N-linked oligosaccharide processing enzyme. J Biol Chem. 1997;272(46):29356-63. DOI:10.1074/jbc.272.46.29356 |
- Hakki Z, Thompson AJ, Bellmaine S, Speciale G, Davies GJ, and Williams SJ. (2015). Structural and kinetic dissection of the endo-α-1,2-mannanase activity of bacterial GH99 glycoside hydrolases from Bacteroides spp. Chemistry. 2015;21(5):1966-77. DOI:10.1002/chem.201405539 |
- Roth J, Ziak M, and Zuber C. (2003). The role of glucosidase II and endomannosidase in glucose trimming of asparagine-linked oligosaccharides. Biochimie. 2003;85(3-4):287-94. DOI:10.1016/s0300-9084(03)00049-x |
- Zuber C, Spiro MJ, Guhl B, Spiro RG, and Roth J. (2000). Golgi apparatus immunolocalization of endomannosidase suggests post-endoplasmic reticulum glucose trimming: implications for quality control. Mol Biol Cell. 2000;11(12):4227-40. DOI:10.1091/mbc.11.12.4227 |
- Dale MP, Kopfler WP, Chait I, and Byers LD. (1986). Beta-glucosidase: substrate, solvent, and viscosity variation as probes of the rate-limiting steps. Biochemistry. 1986;25(9):2522-9. DOI:10.1021/bi00357a036 |
- Spiro MJ and Spiro RG. (2000). Use of recombinant endomannosidase for evaluation of the processing of N-linked oligosaccharides of glycoproteins and their oligosaccharide-lipid precursors. Glycobiology. 2000;10(5):521-9. DOI:10.1093/glycob/10.5.521 |
- Kukushkin NV, Alonzi DS, Dwek RA, and Butters TD. (2011). Demonstration that endoplasmic reticulum-associated degradation of glycoproteins can occur downstream of processing by endomannosidase. Biochem J. 2011;438(1):133-42. DOI:10.1042/BJ20110186 |
- Iwamoto S, Kasahara Y, Kamei K, Seko A, Takeda Y, Ito Y, and Matsuo I. (2014). Measurement of endo-α-mannosidase activity using a fluorescently labeled oligosaccharide derivative. Biosci Biotechnol Biochem. 2014;78(6):927-36. DOI:10.1080/09168451.2014.910101 |
- Matsuda K, Kurakata Y, Miyazaki T, Matsuo I, Ito Y, Nishikawa A, and Tonozuka T. (2011). Heterologous expression, purification, and characterization of an α-mannosidase belonging to glycoside hydrolase family 99 of Shewanella amazonensis. Biosci Biotechnol Biochem. 2011;75(4):797-9. DOI:10.1271/bbb.100874 |
- Thompson AJ, Williams RJ, Hakki Z, Alonzi DS, Wennekes T, Gloster TM, Songsrirote K, Thomas-Oates JE, Wrodnigg TM, Spreitz J, Stütz AE, Butters TD, Williams SJ, and Davies GJ. (2012). Structural and mechanistic insight into N-glycan processing by endo-α-mannosidase. Proc Natl Acad Sci U S A. 2012;109(3):781-6. DOI:10.1073/pnas.1111482109 |
-
Vogel C, Pohlentz G. Synthesis of α-D-glucopyranosyl-(1,3)-α-D-mannopyranosyl-(1,7)-4-methylumbelliferone, a fluorogenic substrate for endo-α-1,2-mannosidase. J. Carbohydr. Chem. 2000, 19: 1247-1258.