CAZypedia needs your help! We have many unassigned GH, PL, CE, AA, GT, and CBM pages in need of Authors and Responsible Curators.
Scientists at all career stages, including students, are welcome to contribute to CAZypedia. Read more here, and in the 10th anniversary article in Glycobiology.
New to the CAZy classification? Read this first.
*
Consider attending the 15th Carbohydrate Bioengineering Meeting in Ghent, 5-8 May 2024.
Difference between revisions of "Polysaccharide Lyase Family 17"
Emil Stender (talk | contribs) |
Harry Brumer (talk | contribs) (Added DOI links to Acta Chem. Scand., Korean J. Chem. references) |
||
| Line 56: | Line 56: | ||
#Jagtap2014 pmid=24795372 | #Jagtap2014 pmid=24795372 | ||
#Park2014 pmid=24478312 | #Park2014 pmid=24478312 | ||
| − | #Shin2015 Shin, J. W., Lee, O. K., Park, H. H., Kim, H. S., and Lee, E. Y. (2015) Molecular characterization of a novel oligoalginate lyase consisting of AlgL- and heparinase II/III-like domains from Stenotrophomonas maltophilia KJ-2 and its application to alginate saccharification. Korean J. Chem. Eng. 32, 917–924 | + | #Shin2015 Shin, J. W., Lee, O. K., Park, H. H., Kim, H. S., and Lee, E. Y. (2015) Molecular characterization of a novel oligoalginate lyase consisting of AlgL- and heparinase II/III-like domains from Stenotrophomonas maltophilia KJ-2 and its application to alginate saccharification. Korean J. Chem. Eng. 32, 917–924 [http://dx.doi.org/10.1007/s11814-014-0282-1 DOI:10.1007/s11814-014-0282-1] |
#Wang2015 pmid=25335746 | #Wang2015 pmid=25335746 | ||
#Mathieu2018 pmid=29795267 | #Mathieu2018 pmid=29795267 | ||
| − | #Haug1967 Haug, A., Larsen, B., and Smidsrod, O. (1967) Studies on sequence of uronic acid residues in alginic acid. Acta Chem. Scand. 21, 691–704 | + | #Haug1967 Haug, A., Larsen, B., and Smidsrod, O. (1967) Studies on sequence of uronic acid residues in alginic acid. Acta Chem. Scand. 21, 691–704 [http://dx.doi.org/10.3891/acta.chem.scand.21-0691 DOI:10.3891/acta.chem.scand.21-0691] |
| − | #Haug1966 Haug, A., Larsen, B., and Smidsrod, O. (1966) A study of constitution of alginic acid by partial acid hydrolysis. Acta Chem. Scand. 20, 183–190 | + | #Haug1966 Haug, A., Larsen, B., and Smidsrod, O. (1966) A study of constitution of alginic acid by partial acid hydrolysis. Acta Chem. Scand. 20, 183–190 [http://dx.doi.org/10.3891/acta.chem.scand.20-0183 DOI:10.3891/acta.chem.scand.20-0183] |
#Meyer1940 pmid=19870951 | #Meyer1940 pmid=19870951 | ||
#Park2012 pmid=21826589 | #Park2012 pmid=21826589 | ||
Revision as of 08:13, 4 July 2019
This page is currently under construction. This means that the Responsible Curator has deemed that the page's content is not quite up to CAZypedia's standards for full public consumption. All information should be considered to be under revision and may be subject to major changes.
- Author: ^^^Emil Stender^^^
- Responsible Curator: ^^^Birte Svensson^^^
| Polysaccharide Lyase Family 17 | |
| 3D structure | (α/α)6 barrel + anti-parallel β-sheet |
| Mechanism | β-eliminationg |
| Charge neutralizer | Asparagine and histidine |
| Active site residues | known |
| CAZy DB link | |
| http://www.cazy.org/PL17.html | |
Substrate specificities
PL17 contains 2 subfamilies [1] as well as several proteins currently not assigned to any subfamily. Subfamily 2 has been shown to be exolytic alginate lyases [2, 3, 4, 5] with activity for all tree block structures observed [6]. Alginate consisting of 1,4 linked β-D-mannuronic acid and α-L-guluronic acid arranged in poly-mannuronic acid blocks, poly-guluronic acid blocks or poly-mannuronic/guluronic acid blocks [7, 8]. Subfamily 1 has been found to be hyaluroran endo-lyases or poly-glucuronic acid lyases [6]. Hyaluronan consisting of N-acetyl-D-glucoamine and 1,4 linked D-glucoronic acid [9].
Kinetics and Mechanism
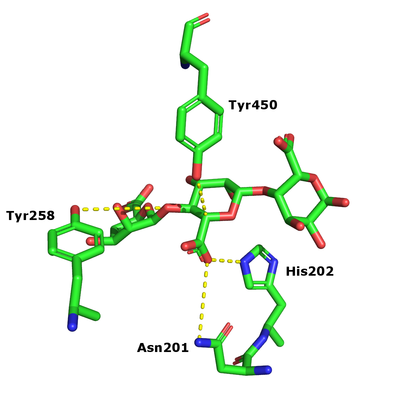
The β-elimination catalyzed by the PL17 enzymes results in the formation of a C4-C5 unsaturated sugar at the new non-reducing end. The first step is the neutralization of the acid group in the +1 subsite by the conserved histidine and asparagine. This lowers the pKa value of the C5-proton allowing for abstraction by the catalytic base (Figure 1). A catalytic acid then donates a proton to the glycosidic linkage resulting in the β-elimination [3].
Catalytic Residues
After charge neutralization a tyrosine functions as the catalytic base and another tyrosine the acid. They were originally identified as Y456 and Y258 in Alg17c from Saccharophagus degradans [3].
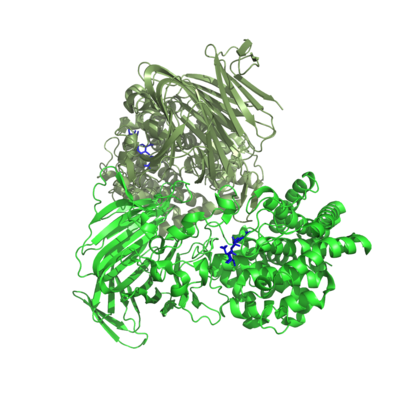
Three-dimensional structures
One crystal structure is available in PL17, that of Alg17c from Saccharophagus degradans belonging to subfamily 2 [3]. It is an (α/α)6 barrel + anti-parallel β-sheet with the catalytic machinery located in the (α/α)6 barrel (Figure 2). Alg17c is a homodimer, though that does not appear to be a general feature of PL17 [2, 3, 4, 5].
Family Firsts
- First catalytic activity
- MJ-3 alginate lyase [10].
- First catalytic base/acid
- Alg17c crystal structure [3]
- First charge neutralizer
- Alg17c crystal structure [3]
- First 3-D structure
- Alg17c crystal structure [3]
References
- Lombard V, Bernard T, Rancurel C, Brumer H, Coutinho PM, and Henrissat B. (2010). A hierarchical classification of polysaccharide lyases for glycogenomics. Biochem J. 2010;432(3):437-44. DOI:10.1042/BJ20101185 |
- Jagtap SS, Hehemann JH, Polz MF, Lee JK, and Zhao H. (2014). Comparative biochemical characterization of three exolytic oligoalginate lyases from Vibrio splendidus reveals complementary substrate scope, temperature, and pH adaptations. Appl Environ Microbiol. 2014;80(14):4207-14. DOI:10.1128/AEM.01285-14 |
- Park D, Jagtap S, and Nair SK. (2014). Structure of a PL17 family alginate lyase demonstrates functional similarities among exotype depolymerases. J Biol Chem. 2014;289(12):8645-55. DOI:10.1074/jbc.M113.531111 |
-
Shin, J. W., Lee, O. K., Park, H. H., Kim, H. S., and Lee, E. Y. (2015) Molecular characterization of a novel oligoalginate lyase consisting of AlgL- and heparinase II/III-like domains from Stenotrophomonas maltophilia KJ-2 and its application to alginate saccharification. Korean J. Chem. Eng. 32, 917–924 DOI:10.1007/s11814-014-0282-1
- Wang L, Li S, Yu W, and Gong Q. (2015). Cloning, overexpression and characterization of a new oligoalginate lyase from a marine bacterium, Shewanella sp. Biotechnol Lett. 2015;37(3):665-71. DOI:10.1007/s10529-014-1706-z |
- Mathieu S, Touvrey-Loiodice M, Poulet L, Drouillard S, Vincentelli R, Henrissat B, Skjåk-Bræk G, and Helbert W. (2018). Ancient acquisition of "alginate utilization loci" by human gut microbiota. Sci Rep. 2018;8(1):8075. DOI:10.1038/s41598-018-26104-1 |
-
Haug, A., Larsen, B., and Smidsrod, O. (1967) Studies on sequence of uronic acid residues in alginic acid. Acta Chem. Scand. 21, 691–704 DOI:10.3891/acta.chem.scand.21-0691
-
Haug, A., Larsen, B., and Smidsrod, O. (1966) A study of constitution of alginic acid by partial acid hydrolysis. Acta Chem. Scand. 20, 183–190 DOI:10.3891/acta.chem.scand.20-0183
- Meyer K, Hobby GL, Chaffee E, and Dawson MH. (1940). THE HYDROLYSIS OF HYALURONIC ACID BY BACTERIAL ENZYMES. J Exp Med. 1940;71(2):137-46. DOI:10.1084/jem.71.2.137 |
- Park HH, Kam N, Lee EY, and Kim HS. (2012). Cloning and characterization of a novel oligoalginate lyase from a newly isolated bacterium Sphingomonas sp. MJ-3. Mar Biotechnol (NY). 2012;14(2):189-202. DOI:10.1007/s10126-011-9402-7 |