CAZypedia needs your help! We have many unassigned GH, PL, CE, AA, GT, and CBM pages in need of Authors and Responsible Curators.
Scientists at all career stages, including students, are welcome to contribute to CAZypedia. Read more here, and in the 10th anniversary article in Glycobiology.
New to the CAZy classification? Read this first.
*
Consider attending the 15th Carbohydrate Bioengineering Meeting in Ghent, 5-8 May 2024.
Difference between revisions of "Polysaccharide Lyase Family 7"
| Line 76: | Line 76: | ||
;First 3-D holo-structure: A1-II from ''Sphingomons'' sp. A1 <cite>Ogura2007</cite> | ;First 3-D holo-structure: A1-II from ''Sphingomons'' sp. A1 <cite>Ogura2007</cite> | ||
| − | ;First CBM: | + | ;First CBM: PL7 and CBM13 from ''Agarivorans'' sp. L11 <cite>Li2015</cite> |
| − | <cite>Li2015</cite> | ||
== References == | == References == | ||
Revision as of 01:09, 5 August 2019
This page is currently under construction. This means that the Responsible Curator has deemed that the page's content is not quite up to CAZypedia's standards for full public consumption. All information should be considered to be under revision and may be subject to major changes.
- Authors: ^^^Nadine Gerlach^^^ and ^^^Jan-Hendrik Hehemann^^^
- Responsible Curator: ^^^Jan-Hendrik Hehemann^^^
| Polysaccharide Lyase Family PL7 | |
| 3D Structure | β jelly roll |
| Mechanism | β-elimination |
| Active site residues | known |
| CAZy DB link | |
| http://www.cazy.org/PL7.html | |
Mechanism
Alginate lyases (Alys) of all families, PL5-7, PL14-15, PL17-18, cleave the glycosidic bond via β-elimination. Most PL7s are endo-active, i.e. acting within a poly- or oligosaccharide and releasing smaller alginate fragments. exo activity [1].
3 steps: [2] In both activity modes, a new nonreducing end with a 4-deoxy-L-erythro-hex-4 en pyranosyl uronate residue (Δ) is formed (Figure 1). In contrast to terrestrial PL7, marine PLs need the divalent cation calcium for substrate recognition and binding [1]. Ca2+ is weakening the ionic interactions between substrate (polyanion) and PL7 (polycation) by reducing the surface density of the alginate charge and therefore increasing the enzyme activity [3].
Kinetics and catalytic residues
Structural and biochemical analyses of wild type and mutated PL7s revealed five residues forming the active site: arginine (R), glutamine (Q), histidine (H), tyrosine (Y) [5], which are assembled in three highly conserved regions (Figure 2). Histidine removes the negative charge of alginate, while
However, there can also be additional charged residues at the active site, which promote substrate recognition and binding [1]. such residues can be found in the N-terminal R*ELREML and VIIGQIH regions. Both highly conserved regions were mainly characterized by hydrophobic amino acids (especially aromatic amino acids) such as leucine, tryptophan and methionine as well as residues with planar polar side chains (especially amino acids with charged side chains) such as arginine, glutamic acid, glutamine (Figure 2). These residues have been suggested to be substrate-binding molecules [6].
Substrate specificities
The polysaccharide lyase family 7 (PL7) contains five subfamilies (SF) based on their sequence similarities [7], plus a so far uncharacterized sixth subfamily, which consist only of marine representatives of the Flavobacteriaceae [1]. All characterized PL7 enzymes were alginate lyases specific for the anionic, gel forming polysaccharide alginate. The substrate specificity depends on the source of alginate, i.e. derived from brown seaweed or mucoid bacteria Pseudomonas spp. and Azotobacter vinelandii, as well as geographical and saisonal parameters. Alginate is an heteropolysaccharide, consisting of β-D-mannuronate (M) and α-L-guluronate (G). These monosaccharides can occur in homogenous and heterogenous blocks. Hence, PL7 lyases can be mannuronate (EC 4.2.2.3), guluronate (EC 4.2.2.11) or mixed link (EC 4.2.2.-) specific. Despite the preference for M- or G-enriched blocks, most PL7s also have a moderate to low processivity for the other building block [1],[8],[9].
SF3 & SF5 G-specific [1] [10, 11]
Substitution of hydrophobic amino acids in the isoleucine site of domain QIH could have an enormous influence on the high affinity to polyM or polyG. This isoleucine was reconfirmed to be indispensable for recognition of the pG or G-G bond [12]
Three-dimensional structures
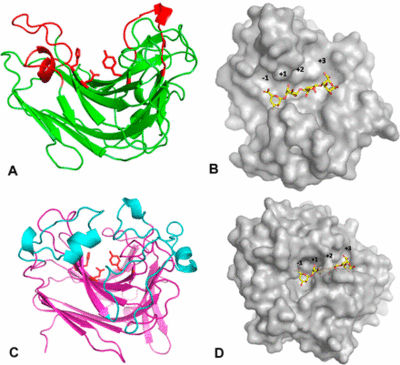
PL 7 is a very well biochemical characterized family with almost 40 entries in the CAZy data base [14]. Structural insights on the other hand are still restricted with nine 3D structures from only eight bacterial strains (status at CAZy in August 2019). The first structure of a PL7 was determined from Pseudomonas aeruginosa by multiple isomorphous replacement (MIR) at 2.0 Å resolution [15]. Just like PL14, PL7 belongs to the jelly roll family with a wide open cleft harboring the active site (Figure 3A, B). Til date, there is only one known exoactive PL7 structure [1].. Zobellia galaactinovorans DsijT is harboring, among others, two PL7 with two completely different activity modifs. AlyA1 belongs to SF3 and is an endo-active PL7. AlyA5 on the other hand belongs to SF5 and is exo-active, which active site is close by three additional loops forming a small pocket (Figure 3C, D).
The highly conserved 9-amino-acid-block YFKAGVY*Q, where * is a variable residue, at the C-terminus of PL7s (Figure 2) has also been found for an extracellular pectate lyase in E. chrysanthemi (Keen & Tamaki, 1986) . Alginate and pectate / pectin lyases share several features such as β-elimination, the recognition of substrates of a similar structure and primary sequence similarity, indicating that they probably share a similar core structural fold. Since alginate lyases and pectinases differ in substrate specificity, it is likely not related to substrate recognition, but rather to maintaining a stable 3D-conformation [6].
Several alginate lyases have been reported to be multimodula domain enzymes with putative non-catalytic, carbohydrate-binding modules (CBMs). However, their exact role has been barely investigated yet. The first biochemically characterized CBM of an endo PL7 was a N-terminal CBM13 from Agarivorans sp. L11, which not only increased its substrate binding ability and therefore catalytic efficiency, but also substrate preference and product distribution as well as thermostability [16]. Contradictory observations regarding catalytic effiency and substrate specificity were made for an endo PL7 from Vibrio splendidus OU02 DNA with an N-terminal CBM32 linked by a unique alpha helix linker [17]. Nevertheless, the CBM and und linker were supposed to serve as "pivont point" affeting the product distribution towards trisaccharides. The PL7 from Persicobacter sp. CCB-QB2 is even consisting of three domains - a N-terminal CBM16 with still unclear function, C-terminal catalytic domain and a CBM32, which is located between both domains [9]. This CBM32 is also not enhancing the catalytic activity and is not binding alginate, but the cleaved termini during catalysis. The crystal structures revealed an arginine residue which is possibly binding to the carboxylic group and a conserved Ca2+ binding site most likely essential for the maintenance of the overall fold.
Evolution of Aly PULs
[18]
Family Firsts
- First catalytic endo-activity
polyM PL7 from Photobacterium ATCC 433367 [19]
polyG PL7 from Klebsiella pneumoniae subbsp. aerogenes [20]
- First catalytic exo-activity
- AlyA5 from Zobellia galactanivorans DsijT [1]
- First 3-D apo-structure
- PA1167 from Pseudomonas aeruginosa [15]
- First 3-D holo-structure
- A1-II from Sphingomons sp. A1 [13]
- First CBM
- PL7 and CBM13 from Agarivorans sp. L11 [16]
References
- Thomas F, Lundqvist LC, Jam M, Jeudy A, Barbeyron T, Sandström C, Michel G, and Czjzek M. (2013). Comparative characterization of two marine alginate lyases from Zobellia galactanivorans reveals distinct modes of action and exquisite adaptation to their natural substrate. J Biol Chem. 2013;288(32):23021-37. DOI:10.1074/jbc.M113.467217 |
- Favorov VV, Vozhova EI, Denisenko VA, and Elyakova LA. (1979). A study of the reaction catalysed by alginate lyase VI from the sea mollusc, Littorina sp. Biochim Biophys Acta. 1979;569(2):259-66. DOI:10.1016/0005-2744(79)90061-5 |
- Yamasaki M, Ogura K, Hashimoto W, Mikami B, and Murata K. (2005). A structural basis for depolymerization of alginate by polysaccharide lyase family-7. J Mol Biol. 2005;352(1):11-21. DOI:10.1016/j.jmb.2005.06.075 |
- Wong TY, Preston LA, and Schiller NL. (2000). ALGINATE LYASE: review of major sources and enzyme characteristics, structure-function analysis, biological roles, and applications. Annu Rev Microbiol. 2000;54:289-340. DOI:10.1146/annurev.micro.54.1.289 |
- Lombard V, Bernard T, Rancurel C, Brumer H, Coutinho PM, and Henrissat B. (2010). A hierarchical classification of polysaccharide lyases for glycogenomics. Biochem J. 2010;432(3):437-44. DOI:10.1042/BJ20101185 |
- Badur AH, Jagtap SS, Yalamanchili G, Lee JK, Zhao H, and Rao CV. (2015). Alginate lyases from alginate-degrading Vibrio splendidus 12B01 are endolytic. Appl Environ Microbiol. 2015;81(5):1865-73. DOI:10.1128/AEM.03460-14 |
- Sim PF, Furusawa G, and Teh AH. (2017). Functional and Structural Studies of a Multidomain Alginate Lyase from Persicobacter sp. CCB-QB2. Sci Rep. 2017;7(1):13656. DOI:10.1038/s41598-017-13288-1 |
-
&Yin2015 pmid=25831216
- Deng S, Ye J, Xu Q, and Zhang H. (2014). Structural and functional studies on three alginate lyases from Vibrio alginolyticus. Protein Pept Lett. 2014;21(2):179-87. DOI:10.2174/09298665113206660094 |
- Lombard V, Golaconda Ramulu H, Drula E, Coutinho PM, and Henrissat B. (2014). The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014;42(Database issue):D490-5. DOI:10.1093/nar/gkt1178 |
- Yamasaki M, Moriwaki S, Miyake O, Hashimoto W, Murata K, and Mikami B. (2004). Structure and function of a hypothetical Pseudomonas aeruginosa protein PA1167 classified into family PL-7: a novel alginate lyase with a beta-sandwich fold. J Biol Chem. 2004;279(30):31863-72. DOI:10.1074/jbc.M402466200 |
- Li S, Yang X, Bao M, Wu Y, Yu W, and Han F. (2015). Family 13 carbohydrate-binding module of alginate lyase from Agarivorans sp. L11 enhances its catalytic efficiency and thermostability, and alters its substrate preference and product distribution. FEMS Microbiol Lett. 2015;362(10). DOI:10.1093/femsle/fnv054 |
- Lyu Q, Zhang K, Zhu Q, Li Z, Liu Y, Fitzek E, Yohe T, Zhao L, Li W, Liu T, Yin Y, and Liu W. (2018). Structural and biochemical characterization of a multidomain alginate lyase reveals a novel role of CBM32 in CAZymes. Biochim Biophys Acta Gen Subj. 2018;1862(9):1862-1869. DOI:10.1016/j.bbagen.2018.05.024 |
- Hehemann JH, Correc G, Barbeyron T, Helbert W, Czjzek M, and Michel G. (2010). Transfer of carbohydrate-active enzymes from marine bacteria to Japanese gut microbiota. Nature. 2010;464(7290):908-12. DOI:10.1038/nature08937 |
- Malissard M, Duez C, Guinand M, Vacheron MJ, Michel G, Marty N, Joris B, Thamm I, and Ghuysen JM. (1993). Sequence of a gene encoding a (poly ManA) alginate lyase active on Pseudomonas aeruginosa alginate. FEMS Microbiol Lett. 1993;110(1):101-6. DOI:10.1111/j.1574-6968.1993.tb06302.x |
- Baron AJ, Wong TY, Hicks SJ, Gacesa P, Willcock D, and McPherson MJ. (1994). Alginate lyase from Klebsiella pneumoniae, subsp. aerogenes: gene cloning, sequence analysis and high-level production in Escherichia coli. Gene. 1994;143(1):61-6. DOI:10.1016/0378-1119(94)90605-x |