CAZypedia celebrates the life of Senior Curator Emeritus Harry Gilbert, a true giant in the field, who passed away in September 2025.
CAZypedia needs your help!
We have many unassigned pages in need of Authors and Responsible Curators. See a page that's out-of-date and just needs a touch-up? - You are also welcome to become a CAZypedian. Here's how.
Scientists at all career stages, including students, are welcome to contribute.
Learn more about CAZypedia's misson here and in this article. Totally new to the CAZy classification? Read this first.
Difference between revisions of "Carbohydrate Binding Module Family 91"
| Line 18: | Line 18: | ||
== Ligand specificities == | == Ligand specificities == | ||
| − | CBM91 from ''Paenibacillus xynaniclasticus'' bound to oat spelt xylan with ''K''<sub>a</sub> value of 2.0×10<sup>-5</sup> M<sup>-1</sup>, and bound birchwood xylan <cite>Ito2022</cite>. It did not bind to lichenan or the cellulosic substrates carboxymethyl-cellulose or ball-milled cellulose <cite>Ito2022</cite>. Therefore, CBM91 can recognize and bind to insoluble xylan <cite>Ito2022</cite>. | + | ''Px''CBM91 from ''Paenibacillus xynaniclasticus'' bound to oat spelt xylan with ''K''<sub>a</sub> value of 2.0×10<sup>-5</sup> M<sup>-1</sup>, and bound birchwood xylan <cite>Ito2022</cite>. It did not bind to lichenan or the cellulosic substrates carboxymethyl-cellulose or ball-milled cellulose <cite>Ito2022</cite>. Therefore, ''Px''CBM91 can recognize and bind to insoluble xylan <cite>Ito2022</cite>. |
== Structural Features == | == Structural Features == | ||
| − | [[Image:The_structure_of_PxXyl43A.png|thumb|300px|right|'''Figure 1. The structure of ''Px''Xyl43A and CBM91 <cite>Ito2023</cite>. | + | [[Image:The_structure_of_PxXyl43A.png|thumb|300px|right|'''Figure 1. The structure of ''Px''Xyl43A and ''Px''CBM91 <cite>Ito2023</cite>. |
| − | '''The structure prediction by Alpha Fold 2 of CBM91 (red). This CBM91 is appended to the catalytic domain of ''Px''Xyl43A (green).]] | + | '''The structure prediction by Alpha Fold 2 of ''Px''CBM91 (red). This CBM91 is appended to the catalytic domain of ''Px''Xyl43A (green).]] |
Alpha Fold 2 structural analysis of ''Px''CBM91 exhibited a β-sandwich fold consisted of 12 β-strands and two opposing antiparallel beta sheets <cite>Ito2023</cite>. The concave surface and loops around it connecting the β-strands possess several hydrophobic amino acid residues, the surface is expected to be the binding site<cite>Ito2023</cite>. | Alpha Fold 2 structural analysis of ''Px''CBM91 exhibited a β-sandwich fold consisted of 12 β-strands and two opposing antiparallel beta sheets <cite>Ito2023</cite>. The concave surface and loops around it connecting the β-strands possess several hydrophobic amino acid residues, the surface is expected to be the binding site<cite>Ito2023</cite>. | ||
== Functionalities == | == Functionalities == | ||
| − | CBM91 often | + | CBM91 are often connected to β-xylosidases belonging to glycoside hydrolase family 43 ([[GH43]]). CBM91 binds to the substrates and would place the catalytic domain in the vicinity of substrates in which substrate concentration is high. These enzymes would utilize CBM91 as a tool for efficient saccharification in combination with other xylanases which release xylobiose and/or xylo-oligosaccharides from insoluble substrates. |
| − | ''Paenibacillus xylaniclastuicus'' was isolated from anaerobic digester fed with pineapple waste and grows with xylose as sole carbon source <cite>Tachaapaikoon2012 Ratanakhanockchai2012</cite>. ''P. xylaniclastuicus'' is supposed to degrade xylosic substrates efficiently because it has a lot of genes encoding xylolytic enzymes. Xylosidases, like GH43, which produce xylose from xylan and xylooligosaccharides and connects to CBM91 is one of vial enzymes. Therefore, CBM91 would contribute to produce the carbon sources for the growth of xylolytic bacteria. | + | ''Paenibacillus xylaniclastuicus'' was isolated from an anaerobic digester fed with pineapple waste and grows with xylose as sole carbon source <cite>Tachaapaikoon2012, Ratanakhanockchai2012</cite>. ''P. xylaniclastuicus'' is supposed to degrade xylosic substrates efficiently because it has a lot of genes encoding xylolytic enzymes. Xylosidases, like GH43, which produce xylose from xylan and xylooligosaccharides and connects to CBM91 is one of vial enzymes. Therefore, CBM91 would contribute to produce the carbon sources for the growth of xylolytic bacteria. |
== Family Firsts == | == Family Firsts == | ||
Revision as of 23:36, 28 July 2025
This page is currently under construction. This means that the Responsible Curator has deemed that the page's content is not quite up to CAZypedia's standards for full public consumption. All information should be considered to be under revision and may be subject to major changes.
| CAZy DB link | |
| https://www.cazy.org/CBM91.html |
Ligand specificities
PxCBM91 from Paenibacillus xynaniclasticus bound to oat spelt xylan with Ka value of 2.0×10-5 M-1, and bound birchwood xylan [1]. It did not bind to lichenan or the cellulosic substrates carboxymethyl-cellulose or ball-milled cellulose [1]. Therefore, PxCBM91 can recognize and bind to insoluble xylan [1].
Structural Features
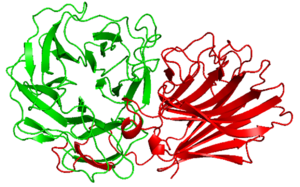
Alpha Fold 2 structural analysis of PxCBM91 exhibited a β-sandwich fold consisted of 12 β-strands and two opposing antiparallel beta sheets [2]. The concave surface and loops around it connecting the β-strands possess several hydrophobic amino acid residues, the surface is expected to be the binding site[2].
Functionalities
CBM91 are often connected to β-xylosidases belonging to glycoside hydrolase family 43 (GH43). CBM91 binds to the substrates and would place the catalytic domain in the vicinity of substrates in which substrate concentration is high. These enzymes would utilize CBM91 as a tool for efficient saccharification in combination with other xylanases which release xylobiose and/or xylo-oligosaccharides from insoluble substrates. Paenibacillus xylaniclastuicus was isolated from an anaerobic digester fed with pineapple waste and grows with xylose as sole carbon source [3, 4]. P. xylaniclastuicus is supposed to degrade xylosic substrates efficiently because it has a lot of genes encoding xylolytic enzymes. Xylosidases, like GH43, which produce xylose from xylan and xylooligosaccharides and connects to CBM91 is one of vial enzymes. Therefore, CBM91 would contribute to produce the carbon sources for the growth of xylolytic bacteria.
Family Firsts
- First Identified
- PxCBM91 from PxXyl43A of Paenibacillus xynaniclasticus strain TW1 [1].
- First Structural Characterization
- β-D-xylosidase, a family 43 glycoside hydrolase from Clostridium acetobutylicum ATCC 824 (Released: 2005-01-25) PDB ID 1Y7B.
References
- Ito D, Nakano E, Karita S, Umekawa M, Ratanakhanokchai K, and Tachaapaikoon C. (2022). Characterization of a GH Family 43 β-Xylosidase Having a Novel Carbohydrate-binding Module from Paenibacillus xylaniclasticus Strain TW1. J Appl Glycosci (1999). 2022;69(3):65-71. DOI:10.5458/jag.jag.JAG-2022_0001 |
-
Ito, D., 2023. Characterization of plant cell wall degrading enzymes from Paenibacillus sp., 2023, Mie University, Ph. D. thesis. https://dl.ndl.go.jp/pid/12910195/1/1
-
C. Tachaapaikoon, S. Tanasupawat, P. Pason, S. Sornyotha, R. Waeonukul, K.L. Kyu and K. Ratanakhanockchai: Paenibacillus xylaniclasticus sp. nov., a xylanolytic-cellulolytic bacterium isolated from sludge in an anaerobic digester. J.Microbiol., 50, 394–400 (2012) DOI:10.1007/s12275-012-1480-3
-
K. Ratanakhanockchai, C. Tachaapaikoon, K.L. Kyu and P. Pason: A novel multienzyme complex from a newly isolated facultative anaerobic bacterium, Paenibacillus sp. TW1. Act. Biol. Hung., 63, 288–300 (2012) DOI:10.1556/ABiol.63.2012.2.10