CAZypedia celebrates the life of Senior Curator Emeritus Harry Gilbert, a true giant in the field, who passed away in September 2025.
CAZypedia needs your help!
We have many unassigned pages in need of Authors and Responsible Curators. See a page that's out-of-date and just needs a touch-up? - You are also welcome to become a CAZypedian. Here's how.
Scientists at all career stages, including students, are welcome to contribute.
Learn more about CAZypedia's misson here and in this article. Totally new to the CAZy classification? Read this first.
Difference between revisions of "Polysaccharide Lyase Family 20"
Harry Brumer (talk | contribs) |
Harry Brumer (talk | contribs) m |
||
| Line 42: | Line 42: | ||
== Three-dimensional structures == | == Three-dimensional structures == | ||
| − | [[Image:PL20.png|thumb|300px|right|'''Figure 1:''' PL20 glucuronan lyase from ''Trichoderma reesei'' ( | + | [[Image:PL20.png|thumb|300px|right|'''Figure 1:''' PL20 glucuronan lyase from ''Trichoderma reesei'' (PDB ID [{{PDBlink}}2zzj 2zzj]).]] |
The ligand-free structure of TrGL was the first PL20 structure to be reported ('''Figure 1''', PDB ID [{{PDBlink}}2zzj 2zzj], 1.8 Å resolution) <cite>Konno2009b</cite>. TrGL has a typical β-jelly roll fold. A calcium binding site, which appears to contribute to the stability, was found at a position far from the cleft. However, no calcium binding site in the cleft has been identified. | The ligand-free structure of TrGL was the first PL20 structure to be reported ('''Figure 1''', PDB ID [{{PDBlink}}2zzj 2zzj], 1.8 Å resolution) <cite>Konno2009b</cite>. TrGL has a typical β-jelly roll fold. A calcium binding site, which appears to contribute to the stability, was found at a position far from the cleft. However, no calcium binding site in the cleft has been identified. | ||
Revision as of 14:20, 6 August 2020
This page has been approved by the Responsible Curator as essentially complete. CAZypedia is a living document, so further improvement of this page is still possible. If you would like to suggest an addition or correction, please contact the page's Responsible Curator directly by e-mail.
- Author: ^^^Naotake Konno^^^
- Responsible Curator: ^^^Shinya Fushinobu^^^
| Polysaccharide Lyase Family PL20 | |
| 3D Structure | β-jelly roll |
| Mechanism | β-elimination |
| Charge neutraliser | none |
| Active site residues | unknown |
| CAZy DB link | |
| https://www.cazy.org/PL20.html | |
Substrate specificities
Polysaccharide lyases of family 20 cleave β-1,4 linkages in polyglucuronate (β-1,4-glucuronan lyase; EC 4.2.2.14). The first PL20 enzyme was cloned from filamentous fungus Trichoderma reesei (TrGL) [1]. TrGL was highly specific for β-1,4-glucuronan prepared from regenerated cellulose by 2,2,6,6-tetramethylpiperidine-1-oxyl radical (TEMPO)-mediated oxidation (cellouronate). Polyglucuronate is a minor polysaccharide compared with other polyuronates. But β-1,4-glucuronan structures are found in water-soluble polysaccharides from bacteria, fungi, and algae.
Kinetics and Mechanism
TrGL has been characterized as a β-1,4-glucuronan lyase; TrGL catalyzed endolytic depolymerization of β-1,4-glucuronan by β-elimination [1]. The enzyme was most active at pH 6.5 and 50°C, and its activity and thermostability increased in the presence of calcium ions.
Catalytic Residues
There are approximately 40 completely conserved amino-acid residues in PL20 members [2]. Possible catalytic residues have been predicted based on structural comparison between TrGL and PL7 alginate lyase A1–II’ [2, 3]. The charge neutralizer, the catalytic base, and the catalytic acid in TrGL are predicted to be Gln91, His53 and Tyr200, respectively. However, in order to clarify the substrate recognition mechanism and the identity of the catalytic residues of PL20, further studies will be required.
Three-dimensional structures
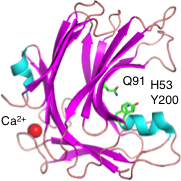
The ligand-free structure of TrGL was the first PL20 structure to be reported (Figure 1, PDB ID 2zzj, 1.8 Å resolution) [2]. TrGL has a typical β-jelly roll fold. A calcium binding site, which appears to contribute to the stability, was found at a position far from the cleft. However, no calcium binding site in the cleft has been identified.
Family Firsts
- First catalytic activity
- TrGl from Trichoderma reesei [1].
- First 3-D structure
- TrGl from Trichoderma reesei [2].
References
- Konno N, Igarashi K, Habu N, Samejima M, and Isogai A. (2009). Cloning of the Trichoderma reesei cDNA encoding a glucuronan lyase belonging to a novel polysaccharide lyase family. Appl Environ Microbiol. 2009;75(1):101-7. DOI:10.1128/AEM.01749-08 |
- Konno N, Ishida T, Igarashi K, Fushinobu S, Habu N, Samejima M, and Isogai A. (2009). Crystal structure of polysaccharide lyase family 20 endo-beta-1,4-glucuronan lyase from the filamentous fungus Trichoderma reesei. FEBS Lett. 2009;583(8):1323-6. DOI:10.1016/j.febslet.2009.03.034 |
- Ogura K, Yamasaki M, Mikami B, Hashimoto W, and Murata K. (2008). Substrate recognition by family 7 alginate lyase from Sphingomonas sp. A1. J Mol Biol. 2008;380(2):373-85. DOI:10.1016/j.jmb.2008.05.008 |