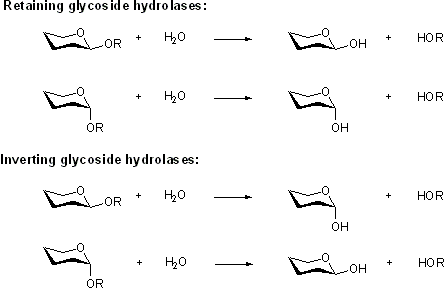CAZypedia celebrates the life of Senior Curator Emeritus Harry Gilbert, a true giant in the field, who passed away in September 2025.
CAZypedia needs your help!
We have many unassigned pages in need of Authors and Responsible Curators. See a page that's out-of-date and just needs a touch-up? - You are also welcome to become a CAZypedian. Here's how.
Scientists at all career stages, including students, are welcome to contribute.
Learn more about CAZypedia's misson here and in this article. Totally new to the CAZy classification? Read this first.
Difference between revisions of "Glycoside Hydrolases"
| Line 41: | Line 41: | ||
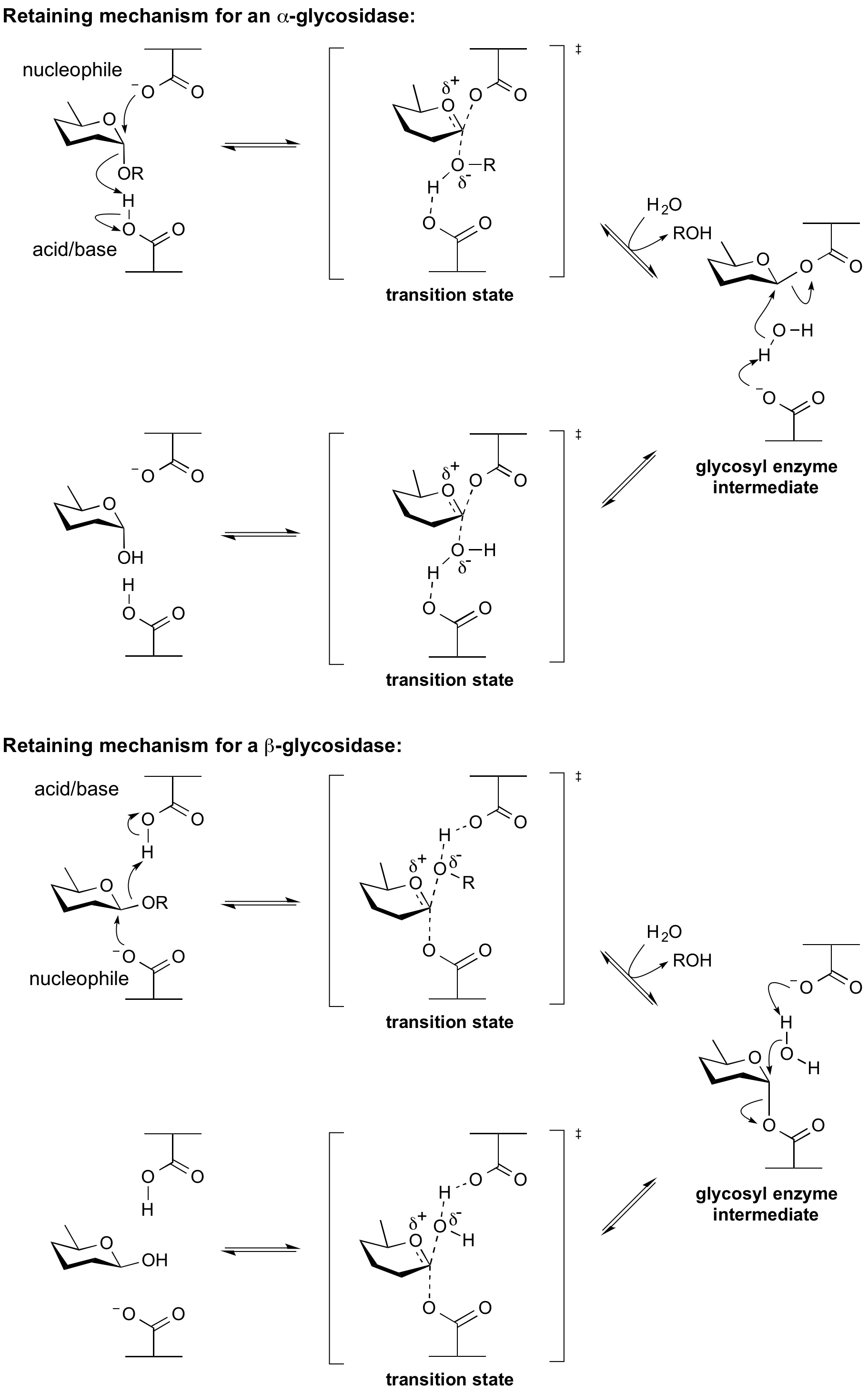
Hydrolysis with net retention of configuration is most commonly achieved via a two step, double-displacement mechanism involving a covalent glycosyl-enzyme [[intermediate]], as is shown in Figure 3. Each step passes through an [[oxocarbenium ion]]-like transition state. Reaction occurs with acid/base assistance provided by two amino acid side chains, typically glutamate or aspartate, located 5.5 A apart. In the first step (often called the glycosylation step), one residue plays the role of a nucleophile, attacking the anomeric centre to displace the aglycon and form a glycosyl enzyme intermediate. At the same time the other residue functions as an [[acid catalyst]] and protonates the glycosidic oxygen as the bond cleaves. In the second step (known as the deglycosylation step), the glycosyl enzyme is hydrolyzed by water, with the other residue now acting as a [[base catalyst]] deprotonating the water molecule as it attacks. The pKa value of the acid/base group cycles between high and low values during catalysis to optimize it for its role at each step of catalysis. | Hydrolysis with net retention of configuration is most commonly achieved via a two step, double-displacement mechanism involving a covalent glycosyl-enzyme [[intermediate]], as is shown in Figure 3. Each step passes through an [[oxocarbenium ion]]-like transition state. Reaction occurs with acid/base assistance provided by two amino acid side chains, typically glutamate or aspartate, located 5.5 A apart. In the first step (often called the glycosylation step), one residue plays the role of a nucleophile, attacking the anomeric centre to displace the aglycon and form a glycosyl enzyme intermediate. At the same time the other residue functions as an [[acid catalyst]] and protonates the glycosidic oxygen as the bond cleaves. In the second step (known as the deglycosylation step), the glycosyl enzyme is hydrolyzed by water, with the other residue now acting as a [[base catalyst]] deprotonating the water molecule as it attacks. The pKa value of the acid/base group cycles between high and low values during catalysis to optimize it for its role at each step of catalysis. | ||
| − | [[Image:Retaining.png|centre]] | + | [[Image:Retaining glycosidase mechanism.png|centre]] |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== References == | == References == | ||
Revision as of 03:48, 9 July 2009
- Author: Spencer Williams
- Responsible Editor: Harry Brumer
Overview
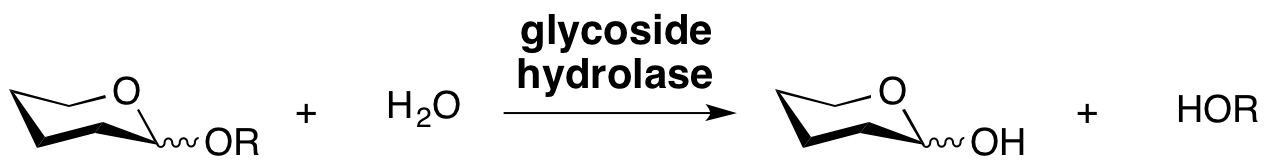
Glycoside hydrolases are enzymes that catalyze the hydrolysis of the glycosidic linkage of glycosides, leading to the formation of a sugar hemiacetal or hemiketal and the corresponding free aglycon. Glycoside hydrolases are also referred to as glycosidases. Glycoside hydrolases can catalyze the hydrolysis of O-, N- and S-linked glycosides.
Classification
Glycoside hydrolases can be classified in many different ways. The following paragraphs list several different ways, the utility of which depends on the context in which the classification is made and used.
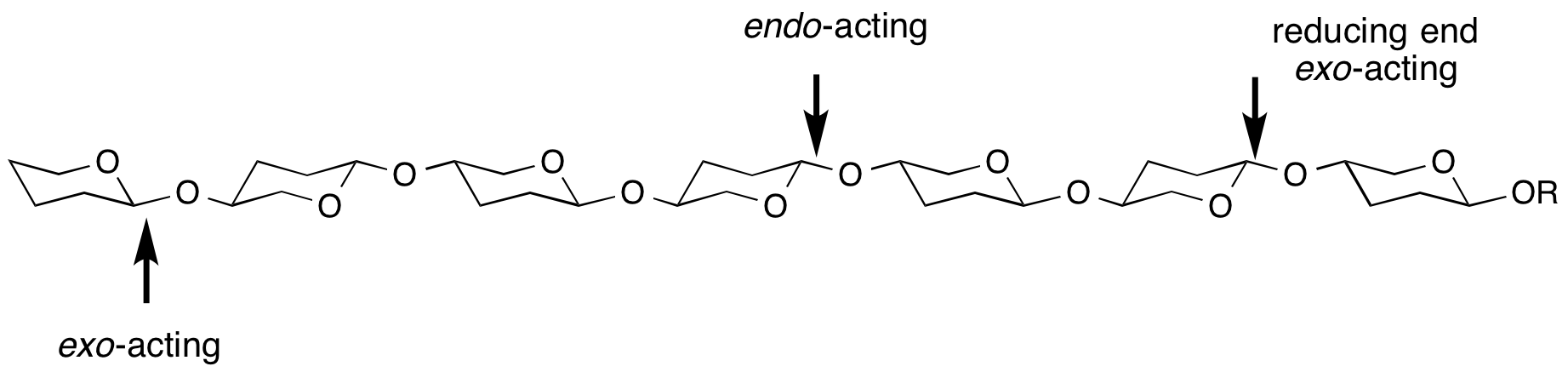
Endo/exo
exo- and endo- refers to the ability of a glycoside hydrolase to cleave a substrate at the end (most frequently, but not always the non-reducing end) or within the middle of a chain. For example, most cellulases are endo-acting, whereas LacZ β-galactosidase from E. coli is exo-acting.
Enzyme Commission (EC) number
EC numbers are codes representing the Enzyme Commission number. This is a numerical classification scheme for enzymes, based on the chemical reactions they catalyze. Every EC number is associated with a recommended name for the respective enzyme. EC numbers do not specify enzymes, but enzyme-catalyzed reactions. If different enzymes (for instance from different organisms) catalyze the same reaction, then they receive the same EC number. A necessary consequence of the EC classification scheme is that codes can be applied only to enzymes for which a function has been biochemically identified. Additionally, certain enzymes can catalyze reactions that fall in more than one class. These enzymes must bear more than one EC number.
Mechanistic classification
Glycoside hydrolases may be classified on the basis of their mechanism. The retaining and inverting glycoside hydrolase classification refers to the stereochemical outcome of the hydrolysis reaction catalyzed by the glycoside hydrolase. Retaining enzymes produce a product with the same stereochemistry as the glycoside substrate , and inverting enzymes give a product with the opposite stereochemistry to the glycoside substrate.
Sequence classification
Sequence classification methods require knowledge of at least part of the amino acid sequence for an enzyme. Algorithmic methods are then used to compare sequences, and in the case of the glycoside hydrolases, this has allowed their classification into more than 100 families. Each family (GH family) contains proteins that are related by sequence, and by corollary, fold. Sequence-based classification schemes allow classification of proteins for which no biochemical evidence may have been obtained. An obvious shortcoming of sequence-based classifications are that they can only be applied to enzymes for which sequence information is available. As such sequence based classification methods are rather different (and in many ways complimentary) to the EC method discussed above.
Mechanism
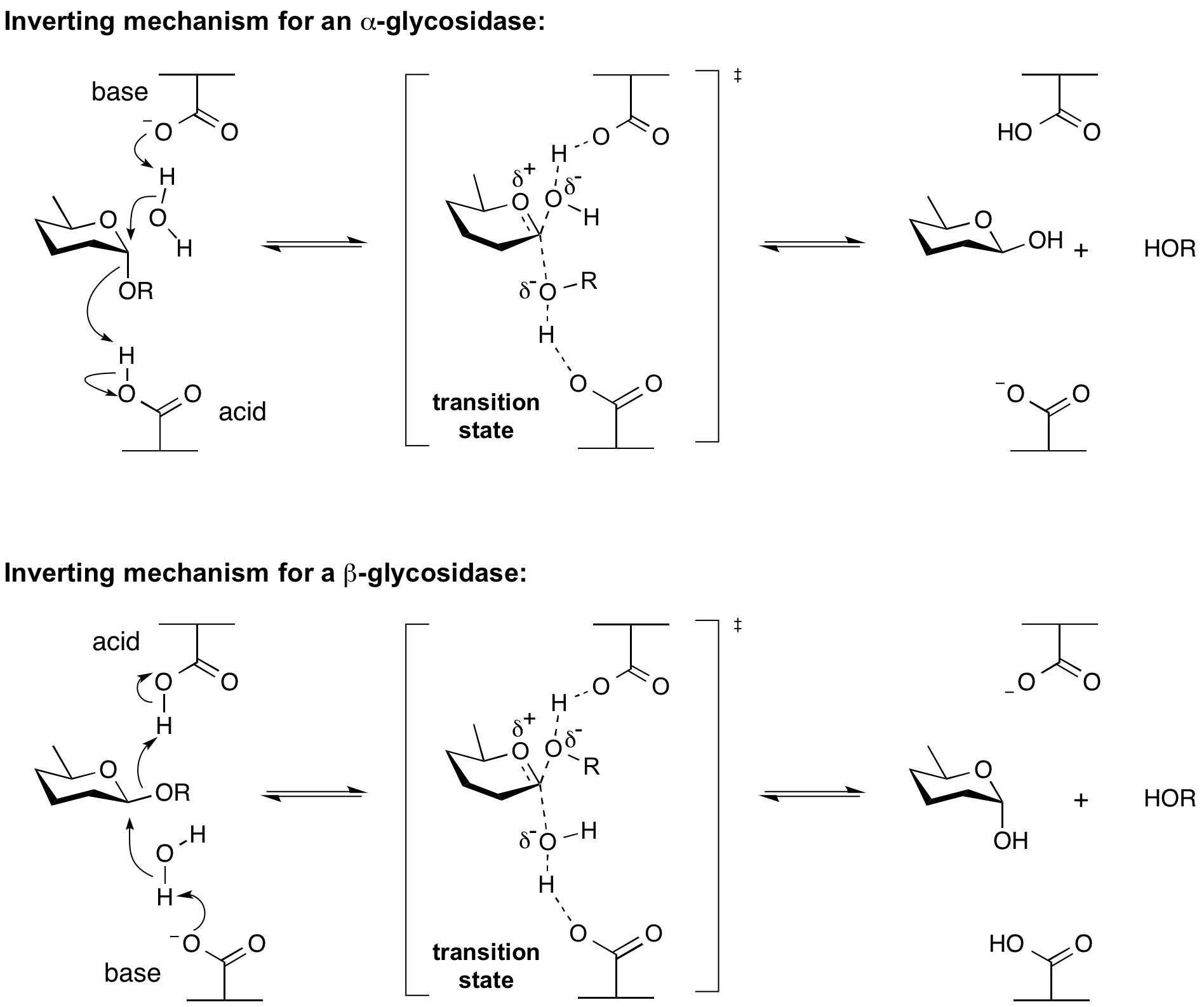
Inverting glycoside hydrolases
Hydrolysis of a glycoside with net inversion of anomeric configuration is generally achieved via a one step, single-displacement mechanism involving oxocarbenium ion-like transition states, as shown below. The reaction occurs with acid/base assistance from two amino acid side chains, normally glutamic or aspartic acids, that are typically located 6-11 A apart.
Retaining glycoside hydrolases
Hydrolysis with net retention of configuration is most commonly achieved via a two step, double-displacement mechanism involving a covalent glycosyl-enzyme intermediate, as is shown in Figure 3. Each step passes through an oxocarbenium ion-like transition state. Reaction occurs with acid/base assistance provided by two amino acid side chains, typically glutamate or aspartate, located 5.5 A apart. In the first step (often called the glycosylation step), one residue plays the role of a nucleophile, attacking the anomeric centre to displace the aglycon and form a glycosyl enzyme intermediate. At the same time the other residue functions as an acid catalyst and protonates the glycosidic oxygen as the bond cleaves. In the second step (known as the deglycosylation step), the glycosyl enzyme is hydrolyzed by water, with the other residue now acting as a base catalyst deprotonating the water molecule as it attacks. The pKa value of the acid/base group cycles between high and low values during catalysis to optimize it for its role at each step of catalysis.