CAZypedia celebrates the life of Senior Curator Emeritus Harry Gilbert, a true giant in the field, who passed away in September 2025.
CAZypedia needs your help!
We have many unassigned pages in need of Authors and Responsible Curators. See a page that's out-of-date and just needs a touch-up? - You are also welcome to become a CAZypedian. Here's how.
Scientists at all career stages, including students, are welcome to contribute.
Learn more about CAZypedia's misson here and in this article. Totally new to the CAZy classification? Read this first.
Difference between revisions of "Polysaccharide Lyase Family 1"
Harry Brumer (talk | contribs) |
Harry Brumer (talk | contribs) |
||
| Line 44: | Line 44: | ||
== Three-dimensional structures == | == Three-dimensional structures == | ||
| − | Pectate lyases PelC (endo-acting) <cite>Yoder1993</cite> and PelE (exo-acting) <cite>Lietzke1994</cite> from ''Erwinia chrysanthemi'', and endo-acting ''Bacillus subtilis'' pectate lyase <cite>Pickersgill1994</cite> (PDB codes: 2PEC, 1PCL, 1BN8). These structures were remarkable in showing the polypeptide chain folded into a right-handed superhelix comprising three β-strands per turn with turns stacking to form a domain of three parallel β-sheets. The structures showed that parallel β-sheets are stable in the absence of protecting α-helices and revealed remarkable side-chain stacks particularly in the hydrophobic interior. Structures of pectin lyase followed <cite>Mayans1997, Vitali1998<cite>, they are closely similar to pectate lyase have the arginine acting as Bronstead base but also have more hydrophobic active centres suitable for binding methylated pectin. | + | Pectate lyases PelC (endo-acting) <cite>Yoder1993</cite> and PelE (exo-acting) <cite>Lietzke1994</cite> from ''Erwinia chrysanthemi'', and endo-acting ''Bacillus subtilis'' pectate lyase <cite>Pickersgill1994</cite> (PDB codes: 2PEC, 1PCL, 1BN8). These structures were remarkable in showing the polypeptide chain folded into a right-handed superhelix comprising three β-strands per turn with turns stacking to form a domain of three parallel β-sheets. The structures showed that parallel β-sheets are stable in the absence of protecting α-helices and revealed remarkable side-chain stacks particularly in the hydrophobic interior. Structures of pectin lyase followed <cite>Mayans1997, Vitali1998</cite>, they are closely similar to pectate lyase have the arginine acting as Bronstead base but also have more hydrophobic active centres suitable for binding methylated pectin. |
== Family Firsts == | == Family Firsts == | ||
Revision as of 18:56, 5 August 2015
This page is currently under construction. This means that the Responsible Curator has deemed that the page's content is not quite up to CAZypedia's standards for full public consumption. All information should be considered to be under revision and may be subject to major changes.
- Author:
- Responsible Curator: ^^^Richard Pickersgill^^^
| Polysaccharide Lyase Family PL1 | |
| 3-D Structure | β-helix |
| Mechanism | β-elimination |
| Charge neutraliser | calcium |
| Active site residues | known |
| CAZy DB link | |
| https://www.cazy.org/PL1.html | |
Substrate specificities
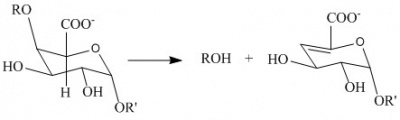
The PL1 family polysaccharide lyases harness anti-β-elimination chemistry to cleave 1,4-linked α-D-galacturonan to produce oligosaccharides with an unsaturated hexenuronic acid residue and a new reducing end [1, 2, 3].
In the figure (Fig. 1) showing pectate lyase activity R and R’ are additional galacturonan residues. Pectin lyases are active against a substrate bearing methyl-ester groups at C6. The geometry of the α1,4-linkage facilitates anti-β-elimination about the C4-glycosidic oxygen bond.
Kinetics and Mechanism
The mechanism involves acidification of the C5 proton of the galacturonan residue binding to the +1 subsite of the enzyme, abstraction of this acidified proton, and subsequent leaving group elimination. The leaving group provides a new reducing end and the group from which it was eliminated has an unsaturated C4-C5 bond. The mechanism of proton abstraction from carbon acids is discussed by Gerlt and Gassman [4].
Catalytic Residues
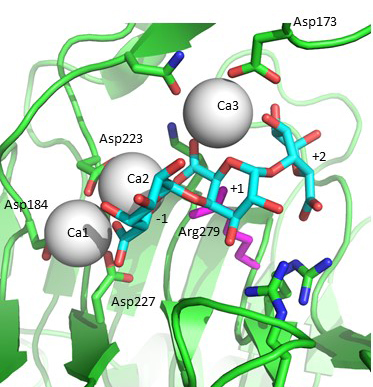
Acidification of the C5 proton in the polysaccharide family 1 enzymes is by the binding of two “catalytic” calcium-ions which are bound only in the Michaelis complex and not to the free enzyme [5, 6, 7]. Abstraction of the C5 proton is by the catalytic arginine, the Bronstead base [5, 6, 7]. The Bacillus subtilis Michaelis complex is shown in Fig. 2. Protonation of the leaving group is probably by solvent water, though other possibilities have been discussed in the literature.
Three-dimensional structures
Pectate lyases PelC (endo-acting) [8] and PelE (exo-acting) [9] from Erwinia chrysanthemi, and endo-acting Bacillus subtilis pectate lyase [10] (PDB codes: 2PEC, 1PCL, 1BN8). These structures were remarkable in showing the polypeptide chain folded into a right-handed superhelix comprising three β-strands per turn with turns stacking to form a domain of three parallel β-sheets. The structures showed that parallel β-sheets are stable in the absence of protecting α-helices and revealed remarkable side-chain stacks particularly in the hydrophobic interior. Structures of pectin lyase followed [11, 12], they are closely similar to pectate lyase have the arginine acting as Bronstead base but also have more hydrophobic active centres suitable for binding methylated pectin.
Family Firsts
- First description of catalytic activity
- Pectin trans-eliminase activity was first demonstrated in 1962 by Albersheim cite>Albersheim1962.
- First catalytic base identification
- Arginine emerged as the catalytic base from the complex of PelC with pentagalacturonate formed using the inactive mutant in which the arginine was substituted by lysine [5, 6], the structure showed that the arginine would be in the correct position to abstract the C5 proton. Comparison of PL10 and PL1 Michaelis complexes cemented the role of the arginine as Bronstead base [13]. More detailed information on specificity emerged from a more recent study [7] which showed the central three subsites bound galacturonsyl-residues, but that the more remote subsites could tolerate methylated galacturonsyl-residues.
- First catalytic divalent cation identification
- The importance of acidifying the C5 proton by stabilizing the charge on the substrate carboxylate was acknowledged early though the conserved arginine was originally thought to fulfil this function [10]. It was later shown that two catalytic calcium-ions bound in the Michaelis complex acidify the C5 proton facilitating its abstraction [5, 6].
- First 3-D structures
- Erwinia chrysanthemi pectate lyases: PelC [8] and PelE [9], and Bacillus subtilis pectate lyase [10].
References
- ALBERSHEIM P and KILLIAS U. (1962). Studies relating to the purification and properties of pectin transeliminase. Arch Biochem Biophys. 1962;97:107-15. DOI:10.1016/0003-9861(62)90050-4 |
- EDSTROM RD and PHAFF HJ. (1964). ELIMINATIVE CLEAVAGE OF PECTIN AND OF OLIGOGALACTURONIDE METHYL ESTERS BY PECTIN TRANS-ELIMINASE. J Biol Chem. 1964;239:2409-15. | Google Books | Open Library
- EDSTROM RD and PHAFF HJ. (1964). PURIFICATION AND CERTAIN PROPERTIES OF PECTIN TRANS-ELIMINASE FROM ASPERGILLUS FONSECAEUS. J Biol Chem. 1964;239:2403-8. | Google Books | Open Library
- Gerlt JA and Gassman PG. (1993). Understanding the rates of certain enzyme-catalyzed reactions: proton abstraction from carbon acids, acyl-transfer reactions, and displacement reactions of phosphodiesters. Biochemistry. 1993;32(45):11943-52. DOI:10.1021/bi00096a001 |
- Scavetta RD, Herron SR, Hotchkiss AT, Kita N, Keen NT, Benen JA, Kester HC, Visser J, and Jurnak F. (1999). Structure of a plant cell wall fragment complexed to pectate lyase C. Plant Cell. 1999;11(6):1081-92. DOI:10.1105/tpc.11.6.1081 |
- Herron SR, Benen JA, Scavetta RD, Visser J, and Jurnak F. (2000). Structure and function of pectic enzymes: virulence factors of plant pathogens. Proc Natl Acad Sci U S A. 2000;97(16):8762-9. DOI:10.1073/pnas.97.16.8762 |
- Seyedarabi A, To TT, Ali S, Hussain S, Fries M, Madsen R, Clausen MH, Teixteira S, Brocklehurst K, and Pickersgill RW. (2010). Structural insights into substrate specificity and the anti beta-elimination mechanism of pectate lyase. Biochemistry. 2010;49(3):539-46. DOI:10.1021/bi901503g |
- Yoder MD, Keen NT, and Jurnak F. (1993). New domain motif: the structure of pectate lyase C, a secreted plant virulence factor. Science. 1993;260(5113):1503-7. DOI:10.1126/science.8502994 |
- Lietzke SE, Yoder MD, Keen NT, and Jurnak F. (1994). The Three-Dimensional Structure of Pectate Lyase E, a Plant Virulence Factor from Erwinia chrysanthemi. Plant Physiol. 1994;106(3):849-862. DOI:10.1104/pp.106.3.849 |
- Pickersgill R, Jenkins J, Harris G, Nasser W, and Robert-Baudouy J. (1994). The structure of Bacillus subtilis pectate lyase in complex with calcium. Nat Struct Biol. 1994;1(10):717-23. DOI:10.1038/nsb1094-717 |
- Mayans O, Scott M, Connerton I, Gravesen T, Benen J, Visser J, Pickersgill R, and Jenkins J. (1997). Two crystal structures of pectin lyase A from Aspergillus reveal a pH driven conformational change and striking divergence in the substrate-binding clefts of pectin and pectate lyases. Structure. 1997;5(5):677-89. DOI:10.1016/s0969-2126(97)00222-0 |
- Vitali J, Schick B, Kester HC, Visser J, and Jurnak F. (1998). The tree-dimensional structure of aspergillus niger pectin lyase B at 1.7-A resolution. Plant Physiol. 1998;116(1):69-80. DOI:10.1104/pp.116.1.69 |
- Charnock SJ, Brown IE, Turkenburg JP, Black GW, and Davies GJ. (2002). Convergent evolution sheds light on the anti-beta -elimination mechanism common to family 1 and 10 polysaccharide lyases. Proc Natl Acad Sci U S A. 2002;99(19):12067-72. DOI:10.1073/pnas.182431199 |
6. Scavetta, R.D., et al., Structure of a plant cell wall fragment complexed to pectate lyase C. Plant Cell, 1999. 11(6): p. 1081-1092.
7. Herron, S.R., et al., Structure and function of pectic enzymes: Virulence factors of plant pathogens. Proceedings of the National Academy of Sciences of the United States of America, 2000. 97(16): p. 8762-8769.
8. Seyedarabi, A., et al., Structural Insights into Substrate Specificity and the anti beta-Elimination Mechanism of Pectate Lyase. Biochemistry, 2010. 49(3): p. 539-546.
9. Yoder, M.D., N.T. Keen, and F. Jurnak, NEW DOMAIN MOTIF - THE STRUCTURE OF PECTATE LYASE-C, A SECRETED PLANT VIRULENCE FACTOR. Science, 1993. 260(5113): p. 1503-1507.
10. Lietzke, S.E., et al., THE 3-DIMENSIONAL STRUCTURE OF PECTATE LYASE-E, A PLANT VIRULENCE FACTOR FROM ERWINIA-CHRYSANTHEMI. Plant Physiology, 1994. 106(3): p. 849-862.
11. Pickersgill, R., et al., THE STRUCTURE OF BACILLUS-SUBTILIS PECTATE LYASE IN COMPLEX WITH CALCIUM. Nature Structural Biology, 1994. 1(10): p. 717-723.
12. Mayans, O., et al., Two crystal structures of pectin lyase A from Aspergillus reveal a pH driven conformational change and striking divergence in the substrate-binding clefts of pectin and pectate lyases. Structure, 1997. 5(5): p. 677-689.
13. Vitali, J., et al., The three-dimensional structure of Aspergillus niger pectin lyase B at 1.7-angstrom resolution. Plant Physiology, 1998. 116(1): p. 69-80.
14. Charnock, S.J., et al., Convergent evolution sheds light on the anti-beta-elimination mechanism common to family 1 and 10 polysaccharide lyases. Proceedings of the National Academy of Sciences of the United States of America, 2002. 99(19): p. 12067-12072.