CAZypedia celebrates the life of Senior Curator Emeritus Harry Gilbert, a true giant in the field, who passed away in September 2025.
CAZypedia needs your help!
We have many unassigned pages in need of Authors and Responsible Curators. See a page that's out-of-date and just needs a touch-up? - You are also welcome to become a CAZypedian. Here's how.
Scientists at all career stages, including students, are welcome to contribute.
Learn more about CAZypedia's misson here and in this article. Totally new to the CAZy classification? Read this first.
Carbohydrate Binding Module Family 1
This page is currently under construction. This means that the Responsible Curator has deemed that the page's content is not quite up to CAZypedia's standards for full public consumption. All information should be considered to be under revision and may be subject to major changes.
- Author: ^^^Markus Linder^^^
- Responsible Curator: ^^^Markus Linder^^^
| CAZy DB link | |
| https://www.cazy.org/CBM1.html |
Ligand specificities
The family 1 CBMs are found mainly in fungal enzymes [1, 2]. Early work showed that family 1 CBMs bind to cellulose [3] and that some, but not all, family 1 CBMs bind to chitin as well [4]. There is also a contribution of CBMs in binding to lignin, but this binding was shown to be non-specific as it was easily blocked by surfactants [5]. Based on NMR measurements it was shown that family 1 CBMs could bind to cellohexaose, but not to shorter cellotriose and cellobiose [6].
Structural Features
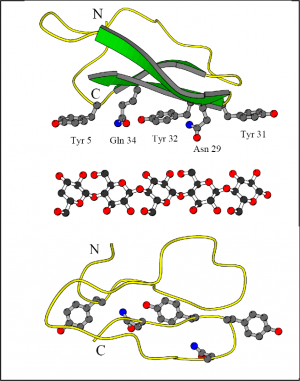
Structurally the family 1 CBMs are distinct from other families. They are relatively small, only about 35 amino acids and have two or three disulphide bridges that stabilize their fold [7]. This type of fold is called a cystine knot and is also found in a family of toxins, called conotoxins produced by cone shells [8]. This structure is rigid and on the CBM there are three aromatic residues (tyrosines or tryptophans) that align so that their spacing is the same as every second pyranose ring on cellulose. Together with some hydrogen bond forming side chains this triad of aromatic residues form a binding face that docks onto the cellulose surface. It has also been shown using synthesised peptides that carbohydrates added to the CMBs affect their binding properties [9]
Functionalities
The CBMs affect enzyme activity by bringing the enzymes close to the cellulose surface[10], but there are also reports that family 1 CBMs can disrupt the crystalline structure of cellulose as well [11]. Family 1 CBMs are found widely in fungal cellulases, also in several enzymes that are not active on cellulose such as mannanase [12] and acetyl xylan esterase [13]. Also swollenins have been found to contain family 1 CBMs [14].
Family 1 CBMs have been used in different types of applications such stabilizing colloid dispersions of drugs by CBM-mediated binding to nanocellulose [15].
Family Firsts
Family 1 CBMs were found first in studies on the Trichoderma reesei Cel7A enzyme (then called cellobiohydrolase I, CBHI) using papain for fragmentation. These studies revealed that Cel7A had a “bifunctional” organization with one part binding strongly to cellulose and the other part containing the catalytic machinery [16]. It was noted that sequences homologous to the smaller cellulose binding part was found in many fungal cellulases and that a synthetic analogue functioned identically to the native fragments produces by proteolysis [3]. The synthetic version of the cellulose binding domain was then analysed by NMR and its structure was determined [7]. With the structure determined the research then led to a number of structure-function studies identifying the amino acids responsible for binding [17] and changing of binding properties by protein engineering [18].
References
- Várnai A, Mäkelä MR, Djajadi DT, Rahikainen J, Hatakka A, and Viikari L. (2014). Carbohydrate-binding modules of fungal cellulases: occurrence in nature, function, and relevance in industrial biomass conversion. Adv Appl Microbiol. 2014;88:103-65. DOI:10.1016/B978-0-12-800260-5.00004-8 |
- Martinez T, Texier H, Nahoum V, Lafitte C, Cioci G, Heux L, Dumas B, O'Donohue M, Gaulin E, and Dumon C. (2015). Probing the Functions of Carbohydrate Binding Modules in the CBEL Protein from the Oomycete Phytophthora parasitica. PLoS One. 2015;10(9):e0137481. DOI:10.1371/journal.pone.0137481 |
-
Johansson, G., Ståhlberg, J., Lindeberg, G., Engström, Å., Pettersson, G. Isolated Fungal Cellulose Terminal Domains and a Synthetic Minimum Analogue Bind to Cellulose. FEBS Lett. 1989; 243, 389–393.
- Linder M, Salovuori I, Ruohonen L, and Teeri TT. (1996). Characterization of a double cellulose-binding domain. Synergistic high affinity binding to crystalline cellulose. J Biol Chem. 1996;271(35):21268-72. DOI:10.1074/jbc.271.35.21268 |
- Palonen H, Tjerneld F, Zacchi G, and Tenkanen M. (2004). Adsorption of Trichoderma reesei CBH I and EG II and their catalytic domains on steam pretreated softwood and isolated lignin. J Biotechnol. 2004;107(1):65-72. DOI:10.1016/j.jbiotec.2003.09.011 |
- Mattinen ML, Linder M, Teleman A, and Annila A. (1997). Interaction between cellohexaose and cellulose binding domains from Trichoderma reesei cellulases. FEBS Lett. 1997;407(3):291-6. DOI:10.1016/s0014-5793(97)00356-6 |
- Kraulis J, Clore GM, Nilges M, Jones TA, Pettersson G, Knowles J, and Gronenborn AM. (1989). Determination of the three-dimensional solution structure of the C-terminal domain of cellobiohydrolase I from Trichoderma reesei. A study using nuclear magnetic resonance and hybrid distance geometry-dynamical simulated annealing. Biochemistry. 1989;28(18):7241-57. DOI:10.1021/bi00444a016 |
- Norton RS and Pallaghy PK. (1998). The cystine knot structure of ion channel toxins and related polypeptides. Toxicon. 1998;36(11):1573-83. DOI:10.1016/s0041-0101(98)00149-4 |
- Happs RM, Guan X, Resch MG, Davis MF, Beckham GT, Tan Z, and Crowley MF. (2015). O-glycosylation effects on family 1 carbohydrate-binding module solution structures. FEBS J. 2015;282(22):4341-56. DOI:10.1111/febs.13500 |
- Igarashi K, Koivula A, Wada M, Kimura S, Penttilä M, and Samejima M. (2009). High speed atomic force microscopy visualizes processive movement of Trichoderma reesei cellobiohydrolase I on crystalline cellulose. J Biol Chem. 2009;284(52):36186-36190. DOI:10.1074/jbc.M109.034611 |
- Hall M, Bansal P, Lee JH, Realff MJ, and Bommarius AS. (2011). Biological pretreatment of cellulose: enhancing enzymatic hydrolysis rate using cellulose-binding domains from cellulases. Bioresour Technol. 2011;102(3):2910-5. DOI:10.1016/j.biortech.2010.11.010 |
- Saloheimo M, Paloheimo M, Hakola S, Pere J, Swanson B, Nyyssönen E, Bhatia A, Ward M, and Penttilä M. (2002). Swollenin, a Trichoderma reesei protein with sequence similarity to the plant expansins, exhibits disruption activity on cellulosic materials. Eur J Biochem. 2002;269(17):4202-11. DOI:10.1046/j.1432-1033.2002.03095.x |
-
Varjonen, S.; Laaksonen, P.; Paananen, A.; Valo, H.; Hähl, H.; Laaksonen, T.; Linder, M. Ben. Self-Assembly of Cellulose Nanofibrils by Genetically Engineered Fusion Proteins. Soft Matter 2011, 7, 2402–2411.
-
van Tilbeurgh, H.; Tomme, P.; Claeyssens, M.; Bhikhabhai, R.; Pettersson, G. Limited Proteolysis of the cellobiohydrolase I from Trichoderma Reesei Separation of Functional Domains. FEBS Lett. 1986; 204, 223–227.
- Linder M, Mattinen ML, Kontteli M, Lindeberg G, Ståhlberg J, Drakenberg T, Reinikainen T, Pettersson G, and Annila A. (1995). Identification of functionally important amino acids in the cellulose-binding domain of Trichoderma reesei cellobiohydrolase I. Protein Sci. 1995;4(6):1056-64. DOI:10.1002/pro.5560040604 |
-
Linder, M.; Nevanen, T.; Teeri, T. T. Design of a pH-Dependent Cellulose-Binding Domain. FEBS Lett. 1999;447,13–16.