CAZypedia celebrates the life of Senior Curator Emeritus Harry Gilbert, a true giant in the field, who passed away in September 2025.
CAZypedia needs your help!
We have many unassigned pages in need of Authors and Responsible Curators. See a page that's out-of-date and just needs a touch-up? - You are also welcome to become a CAZypedian. Here's how.
Scientists at all career stages, including students, are welcome to contribute.
Learn more about CAZypedia's misson here and in this article. Totally new to the CAZy classification? Read this first.
Glycoside Hydrolase Family 136
This page has been approved by the Responsible Curator as essentially complete. CAZypedia is a living document, so further improvement of this page is still possible. If you would like to suggest an addition or correction, please contact the page's Responsible Curator directly by e-mail.
| Glycoside Hydrolase Family GH136 | |
| Clan | GH-N |
| Mechanism | retaining |
| Active site residues | Asp |
| CAZy DB link | |
| https://www.cazy.org/GH136.html | |
Substrate specificities
This family of glycoside hydrolases contains lacto-N-biosidase, as demonstrated for LnbX from Bifidobacterium longum JCM 1217 [1]. LnbX liberated Galβ1-3GlcNAc (lacto-N-biose I, LNB) and lactose from lacto-N-tetraose, the main component of human milk oligosaccharides. It hydrolyzed the linkage GlcNAcβ1-3Gal in lacto-N-hexaose, lacto-N-fucopentaose I, and sialyllacto-N-tetraose a of human milk oligosaccharides as substrate of LnbX in the GH136. In addition, LnbX liberated Galβ1-3GalNAc (GNB) from the sugar chains of globo- and ganglio-series glycosphingolipids [2].
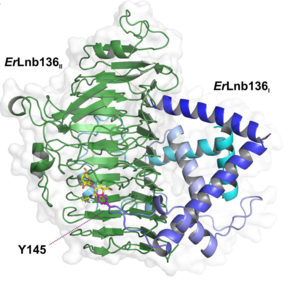
The majority of GH136 lacto-N-biosidases require a neighboring chaperone gene for folding. Rarely, the chaperone-like gene is fused to the lacto-N-biosidase gene, as in case of ErLnb136I and ErLnb136II from Eubacterium ramulus [3].
Kinetics and Mechanism
GH136 lacto-N-biosidases hydrolyze the glycosidic linkage via a anomer-retaining mechanism. The general acid/base catalytic residue of LnbX (Asp411) formed a water-mediated hydrogen bond with the O1 atom of GlcNAc at subsite -1, and a mechanism of Grotthuss proton transfer was proposed [4]. However, subsequent crystallographic reports on three GH136 lacto-N-biosidases ("Er"Lnb136, BsaX, and TnX) revealed a direct hydrogen bond between the general acid/base catalyst and the O1 atom. This observation suggests that a direct proton transfer mechanism is prevalent within this family [3, 5].
Catalytic Residues
For LnbX, the catalytic nucleophile and the catalytic general acid/base are Asp418 and Asp411, respectively.
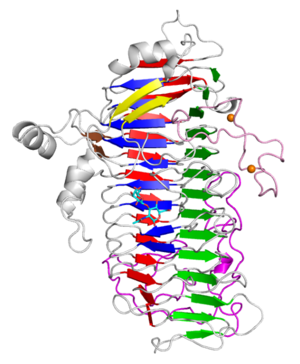
Three-dimensional structures
The X-ray crystal structure of the catalytic domain, LnbXc(31-625) revealed a right-handed β helix fold that is usually shared by polysaccharide-active enzymes. Three forms, ligand free at 2.36 Å resolution (PDB ID 5GQC), LNB complex at 1.82 Å (PDB ID 5GQF), and GNB complex at 2.70 Å (PDB ID 5GQG) were determined [4]. The X-ray crystal structure of ErGH136 in complex with LNB (PDB ID 6KQT) revealed the N-terminal domain (ErLnb136I, from AA 7-224) consists of 8 α-helices (α1-α8) and Y145 of the α6-α7 loop positioned near the active site [3]. The LNB-complexed structures of the catalytic domain of BsaX from Bifidobacterium saguini and TnX from Tyzzerella nexilis were also reported [5].
Family Firsts
- First stereochemistry determination
- LnbX from Bifidobacterium longum [1].
- First catalytic nucleophile identification
- LnbX from Bifidobacterium longum [4].
- First general acid/base residue identification
- LnbX from Bifidobacterium longum [4].
- First 3-D structure
- LnbX from Bifidobacterium longum [4].
References
- Sakurama H, Kiyohara M, Wada J, Honda Y, Yamaguchi M, Fukiya S, Yokota A, Ashida H, Kumagai H, Kitaoka M, Yamamoto K, and Katayama T. (2013). Lacto-N-biosidase encoded by a novel gene of Bifidobacterium longum subspecies longum shows unique substrate specificity and requires a designated chaperone for its active expression. J Biol Chem. 2013;288(35):25194-25206. DOI:10.1074/jbc.M113.484733 |
- Gotoh A, Katoh T, Sugiyama Y, Kurihara S, Honda Y, Sakurama H, Kambe T, Ashida H, Kitaoka M, Yamamoto K, and Katayama T. (2015). Novel substrate specificities of two lacto-N-biosidases towards β-linked galacto-N-biose-containing oligosaccharides of globo H, Gb5, and GA1. Carbohydr Res. 2015;408:18-24. DOI:10.1016/j.carres.2015.03.005 |
- Pichler MJ, Yamada C, Shuoker B, Alvarez-Silva C, Gotoh A, Leth ML, Schoof E, Katoh T, Sakanaka M, Katayama T, Jin C, Karlsson NG, Arumugam M, Fushinobu S, and Abou Hachem M. (2020). Butyrate producing colonic Clostridiales metabolise human milk oligosaccharides and cross feed on mucin via conserved pathways. Nat Commun. 2020;11(1):3285. DOI:10.1038/s41467-020-17075-x |
- Yamada C, Gotoh A, Sakanaka M, Hattie M, Stubbs KA, Katayama-Ikegami A, Hirose J, Kurihara S, Arakawa T, Kitaoka M, Okuda S, Katayama T, and Fushinobu S. (2017). Molecular Insight into Evolution of Symbiosis between Breast-Fed Infants and a Member of the Human Gut Microbiome Bifidobacterium longum. Cell Chem Biol. 2017;24(4):515-524.e5. DOI:10.1016/j.chembiol.2017.03.012 |
- Yamada C, Katayama T, and Fushinobu S. (2022). Crystal structures of glycoside hydrolase family 136 lacto-N-biosidases from monkey gut- and human adult gut bacteria. Biosci Biotechnol Biochem. 2022;86(4):464-475. DOI:10.1093/bbb/zbac015 |