CAZypedia needs your help! We have many unassigned GH, PL, CE, AA, GT, and CBM pages in need of Authors and Responsible Curators.
Scientists at all career stages, including students, are welcome to contribute to CAZypedia. Read more here, and in the 10th anniversary article in Glycobiology.
New to the CAZy classification? Read this first.
*
Consider attending the 15th Carbohydrate Bioengineering Meeting in Ghent, 5-8 May 2024.
Difference between revisions of "Absolute configuration (D/L nomenclature)"
(New page: * Author: Stephen Withers * Responsible Curator: Spencer Williams ---- The absolute configuration of all monosaccharides is denoted by ...) |
|||
| Line 1: | Line 1: | ||
| − | |||
| − | |||
* [[Author]]: [[User:Withers|Stephen Withers]] | * [[Author]]: [[User:Withers|Stephen Withers]] | ||
* [[Responsible Curator]]: [[User:SpencerWilliams|Spencer Williams]] | * [[Responsible Curator]]: [[User:SpencerWilliams|Spencer Williams]] | ||
---- | ---- | ||
| + | |||
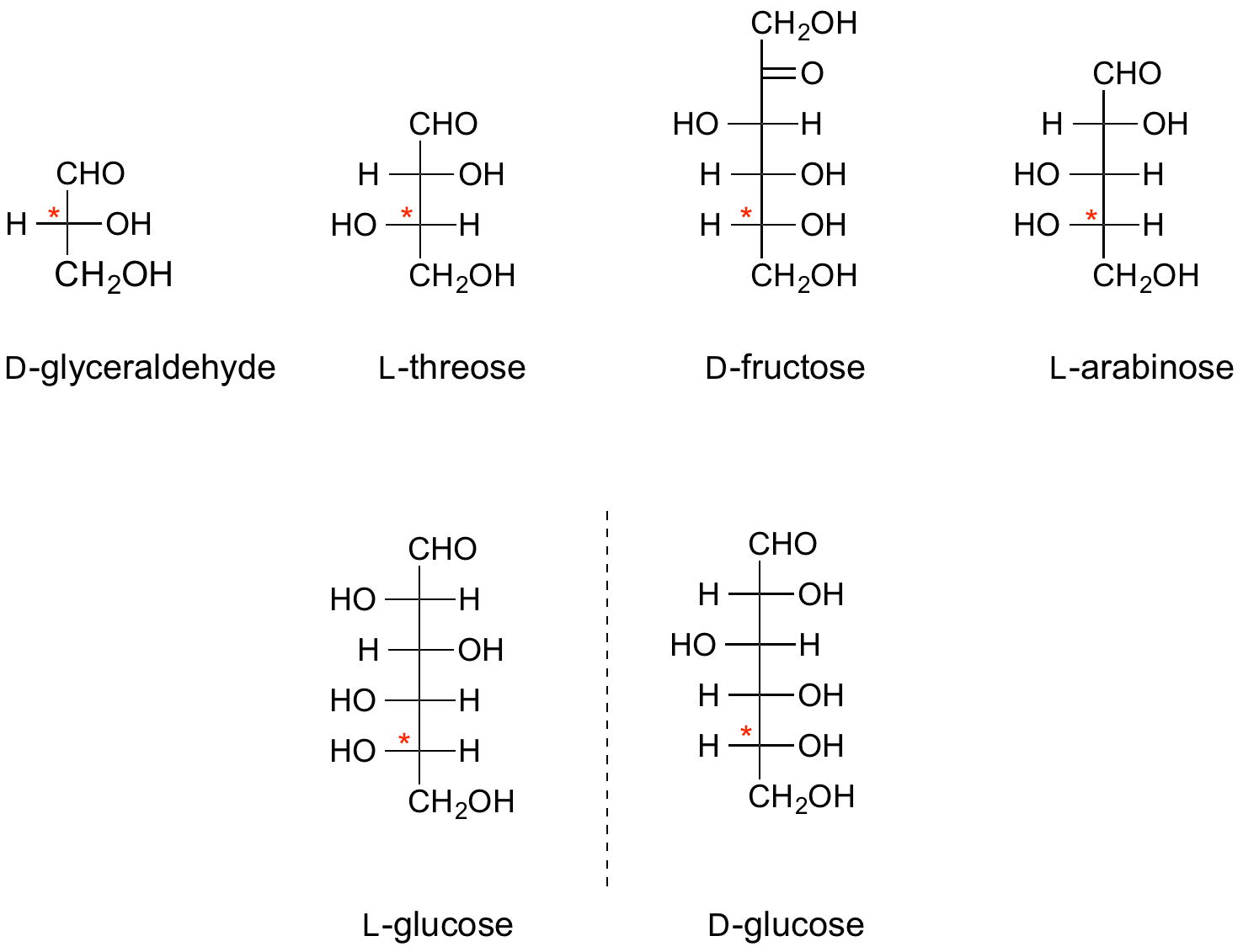
The absolute configuration of all monosaccharides is denoted by the configuration at one particular stereocentre in that sugar, namely the stereocentre furthest from the anomeric centre (the carbonyl carbon in the open chain representation). If, in the Fischer projection, that centre has the hydroxyl group on the right, it is a D-sugar; if on the left, it is an L-sugar. By convention, the "D" and "L" symbols are written in small capitals. | The absolute configuration of all monosaccharides is denoted by the configuration at one particular stereocentre in that sugar, namely the stereocentre furthest from the anomeric centre (the carbonyl carbon in the open chain representation). If, in the Fischer projection, that centre has the hydroxyl group on the right, it is a D-sugar; if on the left, it is an L-sugar. By convention, the "D" and "L" symbols are written in small capitals. | ||
Revision as of 04:25, 16 July 2009
The absolute configuration of all monosaccharides is denoted by the configuration at one particular stereocentre in that sugar, namely the stereocentre furthest from the anomeric centre (the carbonyl carbon in the open chain representation). If, in the Fischer projection, that centre has the hydroxyl group on the right, it is a D-sugar; if on the left, it is an L-sugar. By convention, the "D" and "L" symbols are written in small capitals.
The configurations of the other centres relative to this define the individual sugars, e.g. D-glucose, L-threose, etc.