CAZypedia celebrates the life of Senior Curator Emeritus Harry Gilbert, a true giant in the field, who passed away in September 2025.
CAZypedia needs your help!
We have many unassigned pages in need of Authors and Responsible Curators. See a page that's out-of-date and just needs a touch-up? - You are also welcome to become a CAZypedian. Here's how.
Scientists at all career stages, including students, are welcome to contribute.
Learn more about CAZypedia's misson here and in this article. Totally new to the CAZy classification? Read this first.
Difference between revisions of "Polysaccharide Lyase Family 6"
Emil Stender (talk | contribs) |
Emil Stender (talk | contribs) |
||
| Line 32: | Line 32: | ||
== Substrate specificities == | == Substrate specificities == | ||
| − | PL6 currently contains 3 subfamilies <cite> | + | PL6 currently contains 3 subfamilies <cite>Lombard2010</cite> all of which contain members catalyzing the depolymerisation of alginate <cite>Mathieu2016</cite>. Alginate consisting of 1,4 linked β-D-mannuronic acid and α-L-guluronic acid arranged in poly-mannuronic acid blocks, poly-guluronic acid blocks or poly-mannuronic/guluronic acid blocks <cite>Haug1966 Haug1967</cite>. Subfamily 2 and 3 have so far only shown specificity for poly-mannuronic/guluronic acid blocks <cite>Mathieu2016</cite>, while subfamily 1 has been demonstrated to depolymerize poly-guluronic acid <cite>Lyu2019 Xu2017</cite>, poly-mannuronic acid <cite>Maki1993</cite>, poly-mannuronic/guluronic acid <cite>Mathieu2016</cite> as well as dermatan sulfate (formerly chrondroitin B) <cite>Mathieu2016 #Huang1999 #Michel2004</cite>. |
'' | '' | ||
| Line 53: | Line 53: | ||
== References == | == References == | ||
| − | <biblio>#Lombard2010 pmid=20925655 | + | <biblio> |
| + | #Lombard2010 pmid=20925655 | ||
#Mathieu2016 pmid=27438604 | #Mathieu2016 pmid=27438604 | ||
#Haug1967 Haug, A., Larsen, B., and Smidsrod, O. (1967) Studies on sequence of uronic acid residues in alginic acid. Acta Chem. Scand. 21, 691–704 | #Haug1967 Haug, A., Larsen, B., and Smidsrod, O. (1967) Studies on sequence of uronic acid residues in alginic acid. Acta Chem. Scand. 21, 691–704 | ||
Revision as of 04:07, 6 June 2019
This page is currently under construction. This means that the Responsible Curator has deemed that the page's content is not quite up to CAZypedia's standards for full public consumption. All information should be considered to be under revision and may be subject to major changes.
- Author: ^^^Emil Stender^^^
- Responsible Curator: ^^^Birte Svensson^^^
| Polysaccharide Lyase Family 6 | |
| 3D structure | parralel β-helix |
| Mechanism | β-elimination |
| Charge neutralizer | calcium or water |
| Active site residues | known |
| CAZy DB link | |
| https://www.cazy.org/PL6.html | |
Substrate specificities
PL6 currently contains 3 subfamilies [1] all of which contain members catalyzing the depolymerisation of alginate [2]. Alginate consisting of 1,4 linked β-D-mannuronic acid and α-L-guluronic acid arranged in poly-mannuronic acid blocks, poly-guluronic acid blocks or poly-mannuronic/guluronic acid blocks [3, 4]. Subfamily 2 and 3 have so far only shown specificity for poly-mannuronic/guluronic acid blocks [2], while subfamily 1 has been demonstrated to depolymerize poly-guluronic acid [5, 6], poly-mannuronic acid [7], poly-mannuronic/guluronic acid [2] as well as dermatan sulfate (formerly chrondroitin B) [2, 8, 9].
Kinetics and Mechanism
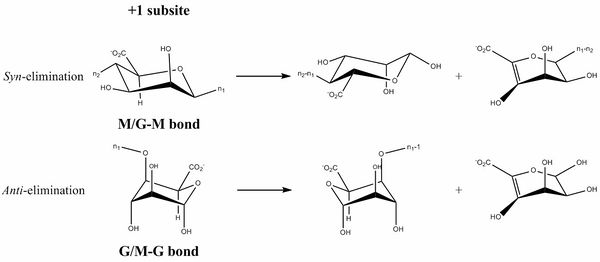
The β-elimination catalyzed by the PL6 enzymes results in the formation of a C4-C5 unsaturated sugar at the new non-reducing end. The first step is the neutralization of the acid group in the +1 subsite by a calcium [6, 9] or by water [5]. This lowers the pKa value of the C5-proton allowing for abstraction by the catalytic base (Figure 1). A catalytic acid then donates a proton to the glycosidic linkage resulting in the β-elimination. This can be done in syn with the acid and base on the same side of the sugar ring in the transition state (the case for D-mannuronic acid) or anti where they are on opposite sides of the sugar ring (the case for L-guluronic acid) [10, 11].
Catalytic Residues
Content is to be added here.
Three-dimensional structures
Content is to be added here.
Family Firsts
- First stereochemistry determination
- Content is to be added here.
- First catalytic nucleophile identification
- Content is to be added here.
- First general acid/base residue identification
- Content is to be added here.
- First 3-D structure
- Content is to be added here.
References
- Lombard V, Bernard T, Rancurel C, Brumer H, Coutinho PM, and Henrissat B. (2010). A hierarchical classification of polysaccharide lyases for glycogenomics. Biochem J. 2010;432(3):437-44. DOI:10.1042/BJ20101185 |
- Mathieu S, Henrissat B, Labre F, Skjåk-Bræk G, and Helbert W. (2016). Functional Exploration of the Polysaccharide Lyase Family PL6. PLoS One. 2016;11(7):e0159415. DOI:10.1371/journal.pone.0159415 |
-
Haug, A., Larsen, B., and Smidsrod, O. (1966) A study of constitution of alginic acid by partial acid hydrolysis. Acta Chem. Scand. 20, 183–190
-
Haug, A., Larsen, B., and Smidsrod, O. (1967) Studies on sequence of uronic acid residues in alginic acid. Acta Chem. Scand. 21, 691–704
- Lyu Q, Zhang K, Shi Y, Li W, Diao X, and Liu W. (2019). Structural insights into a novel Ca(2+)-independent PL-6 alginate lyase from Vibrio OU02 identify the possible subsites responsible for product distribution. Biochim Biophys Acta Gen Subj. 2019;1863(7):1167-1176. DOI:10.1016/j.bbagen.2019.04.013 |
- Xu F, Dong F, Wang P, Cao HY, Li CY, Li PY, Pang XH, Zhang YZ, and Chen XL. (2017). Novel Molecular Insights into the Catalytic Mechanism of Marine Bacterial Alginate Lyase AlyGC from Polysaccharide Lyase Family 6. J Biol Chem. 2017;292(11):4457-4468. DOI:10.1074/jbc.M116.766030 |
- Maki H, Mori A, Fujiyama K, Kinoshita S, and Yoshida T. (1993). Cloning, sequence analysis and expression in Escherichia coli of a gene encoding an alginate lyase from Pseudomonas sp. OS-ALG-9. J Gen Microbiol. 1993;139(5):987-93. DOI:10.1099/00221287-139-5-987 |
- Huang W, Matte A, Li Y, Kim YS, Linhardt RJ, Su H, and Cygler M. (1999). Crystal structure of chondroitinase B from Flavobacterium heparinum and its complex with a disaccharide product at 1.7 A resolution. J Mol Biol. 1999;294(5):1257-69. DOI:10.1006/jmbi.1999.3292 |
- Michel G, Pojasek K, Li Y, Sulea T, Linhardt RJ, Raman R, Prabhakar V, Sasisekharan R, and Cygler M. (2004). The structure of chondroitin B lyase complexed with glycosaminoglycan oligosaccharides unravels a calcium-dependent catalytic machinery. J Biol Chem. 2004;279(31):32882-96. DOI:10.1074/jbc.M403421200 |
- Garron ML and Cygler M. (2010). Structural and mechanistic classification of uronic acid-containing polysaccharide lyases. Glycobiology. 2010;20(12):1547-73. DOI:10.1093/glycob/cwq122 |
- Xu F, Wang P, Zhang YZ, and Chen XL. (2018). Diversity of Three-Dimensional Structures and Catalytic Mechanisms of Alginate Lyases. Appl Environ Microbiol. 2018;84(3). DOI:10.1128/AEM.02040-17 |