CAZypedia needs your help! We have many unassigned GH, PL, CE, AA, GT, and CBM pages in need of Authors and Responsible Curators.
Scientists at all career stages, including students, are welcome to contribute to CAZypedia. Read more here, and in the 10th anniversary article in Glycobiology.
New to the CAZy classification? Read this first.
*
Consider attending the 15th Carbohydrate Bioengineering Meeting in Ghent, 5-8 May 2024.
Difference between revisions of "Glycoside Hydrolase Family 119"
| Line 40: | Line 40: | ||
== Catalytic Residues == | == Catalytic Residues == | ||
| − | ''In silico'' studies have revealed a close phylogenetic relationship between GH families 119 and 57 <cite>Janecek2012,Polacek2023</cite>. Through multiple sequence alignment (MSA) and superimposition of GH119 homology models and [[GH57]] crystallographic structures, it has been demonstrated that GH119 sequences share the same five CSRs typical of [[GH57]] <cite>Janecek2012</cite>. The strictly conserved, catalytic residues of GH57, namely a strand β4 glutamate serving as nucleophile and a β7 aspartate serving as acid/base <cite>Imamura2001,Imamura2003</cite> are located in CSR-3 and CSR-4, respectively. The equivalent residues in CocoGH119 (E225 and D369) have been found to be essential to catalysis by site-directed mutagenesis experiments resulting in complete abolishment of the enzyme’s activity <cite>Vuillemin2024</cite>. Furthermore, homology models of GH119 sequences suggest that their putative catalytic domain may adopt a (β/α)<sub>7</sub>-barrel (i.e., an incomplete TIM-barrel) fold followed by a bundle of α-helices <cite>Janecek2012,Polacek2023</cite>. | + | ''In silico'' studies have revealed a close phylogenetic relationship between GH families 119 and [[57]] <cite>Janecek2012,Polacek2023</cite>. Through multiple sequence alignment (MSA) and superimposition of GH119 homology models and [[GH57]] crystallographic structures, it has been demonstrated that GH119 sequences share the same five CSRs typical of [[GH57]] <cite>Janecek2012</cite>. The strictly conserved, catalytic residues of GH57, namely a strand β4 glutamate serving as nucleophile and a β7 aspartate serving as acid/base <cite>Imamura2001,Imamura2003</cite> are located in CSR-3 and CSR-4, respectively. The equivalent residues in CocoGH119 (E225 and D369) have been found to be essential to catalysis by site-directed mutagenesis experiments resulting in complete abolishment of the enzyme’s activity <cite>Vuillemin2024</cite>. Furthermore, homology models of GH119 sequences suggest that their putative catalytic domain may adopt a (β/α)<sub>7</sub>-barrel (i.e., an incomplete TIM-barrel) fold followed by a bundle of α-helices <cite>Janecek2012,Polacek2023</cite>. |
| − | These characteristics differentiate the amylolytic families [[GH57]] and GH119 from the main α-amylase family, GH13, which adopts a (β/α)<sub>8</sub>-barrel fold (i.e., a classical TIM-barrel) and has a Asp-Glu-Asp catalytic triad, as also observed in GH70 and GH77, all of which are members of clan GH-H <cite>Janecek2022</cite>. Based on these differences, GH119 and [[GH57]] define clan GH-S <cite>Vuillemin2024</cite>. | + | These characteristics differentiate the amylolytic families [[GH57]] and GH119 from the main α-amylase family, [[GH13]], which adopts a (β/α)<sub>8</sub>-barrel fold (i.e., a classical TIM-barrel) and has a Asp-Glu-Asp catalytic triad, as also observed in [[GH70]] and [[GH77]], all of which are members of clan GH-H <cite>Janecek2022</cite>. Based on these differences, GH119 and [[GH57]] define clan GH-S <cite>Vuillemin2024</cite>. |
== Three-dimensional structures == | == Three-dimensional structures == | ||
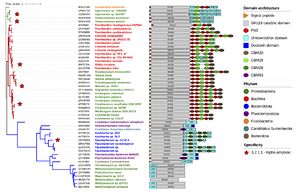
No 3D structure of a GH119 protein has been experimentally established so far. However, domain prediction indicates that several GH119 proteins exhibit a multi-modular architecture consisting of an N-terminal, putatively-catalytic, main domain followed by a variable number of additional domains, including carbohydrate-binding modules (CBM) of families [[CBM20]], CBM25 and CBM26, fibronectin type III (FN-III) and dockerin domains, among others <cite>Polacek2023, Vuillemin2024,Janecek2019</cite>. A phylogenetic tree of the family annotated with predicted domain architecture (Fig. 2) shows that different branches exhibit distinctive auxiliary domain patterns <cite>Vuillemin2024</cite>. Domain prediction for IgtZ suggests that this enzyme comprises an N-terminal, putative catalytic domain, followed by an FN-III, two [[CBM20]] in tandem and a C-terminal CBM25 <cite>Polacek2023</cite>. | No 3D structure of a GH119 protein has been experimentally established so far. However, domain prediction indicates that several GH119 proteins exhibit a multi-modular architecture consisting of an N-terminal, putatively-catalytic, main domain followed by a variable number of additional domains, including carbohydrate-binding modules (CBM) of families [[CBM20]], CBM25 and CBM26, fibronectin type III (FN-III) and dockerin domains, among others <cite>Polacek2023, Vuillemin2024,Janecek2019</cite>. A phylogenetic tree of the family annotated with predicted domain architecture (Fig. 2) shows that different branches exhibit distinctive auxiliary domain patterns <cite>Vuillemin2024</cite>. Domain prediction for IgtZ suggests that this enzyme comprises an N-terminal, putative catalytic domain, followed by an FN-III, two [[CBM20]] in tandem and a C-terminal CBM25 <cite>Polacek2023</cite>. | ||
Revision as of 04:32, 9 February 2024
This page is currently under construction. This means that the Responsible Curator has deemed that the page's content is not quite up to CAZypedia's standards for full public consumption. All information should be considered to be under revision and may be subject to major changes.
| Glycoside Hydrolase Family GH119 | |
| Clan | GH-S |
| Mechanism | retaining |
| Active site residues | known (inferred) |
| CAZy DB link | |
| http://www.cazy.org/GH119.html | |
Substrate specificities
Glycoside hydrolase family 119 (GH119) contains a relatively small number of exclusively bacterial sequences. The first experimentally-characterized member was the amylolytic enzyme IgtZ from Niallia circulans AM7 (formerly Bacillus circulans). Watanabe et al. [1] expressed IgtZ’s full sequence and assayed it against a range of oligo- and polymeric α-glucans, demonstrating its ability to break down amylose and soluble starch but not pullulan or dextran. This suggests a strict specificity towards α-1,4 over α-1,6-glucan bonds. The enzyme also acts on maltooligosaccharides with a minimum degree of polymerization (DP) of 4 and maltooligosyl trehaloses with maltooligosaccharide portions of at least 4 monosaccharide units. The enzyme hydrolytic action yields primarily disaccharides (maltose or trehalose) accompanied by smaller amounts of glucose and other maltodextrins. The enzyme specificity increases with the DP of the substrate. Based on these findings, the authors categorized IgtZ as an α-amylase [1].
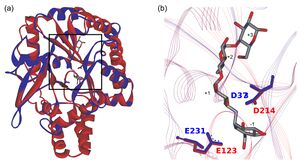
In the CAZy database, the two largest amylolytic families, GH13 and GH57, are notably multi-specific, with α-amylase representing just one of more than 30 specificities in GH13 [2]. Family GH119 was predicted in 2012 [3] to share its catalytic domain fold and catalytic machinery with those of the family GH57 (Fig. 1). It had been also predicted that GH119 would be largely mono-specific, with α-amylase being the primary specificity [4]. This was based on the composition of one of its conserved sequence regions (CSRs), specifically CSR-5, which correlates with substrate specificity in families GH57 and GH119 [5, 6].
Figure 1. Original structure comparison of families GH57 and GH119 from 2012 [3]. (a) Catalytic (β/α)7-barrel with succeeding α-helical bundle of Thermococcus litoralis GH57 4-α-glucanotransferase (red; PDB: 1K1Y; residues M1-Q381) superimposed with substantial part of the (β/α)7-barrel domain of Niallia circulans GH119 α-amylase IgtZ (blue; model; residues T121-D429). The rectangle indicates a detailed view on the right. (b) Focus on catalytic residues in GH57 4-α-glucanotransferase (Glu123 and Asp214) and the predicted catalytic machinery in GH119 α-amylase (Glu231 and Asp373). Acarbose occupying subsites -1 through +3 from the complex with GH57 4-α-glucanotransferase is also shown.
Vuillemin et al. [7] expressed recombinantly the core domain of five other GH119 sequences representing the major phylogenetic clades of family GH119. They all showed a very similar specificity as IgtZ’s: activity on glycogen, soluble starch, amylose and maltooligosaccharides with minimum DP5, but not on pullulan or dextran. This result confirmed the in silico prediction of uniform specificity. Their product profiles were also similar to IgtZ’s, except for that of α-amylase CocoGH119 from Corallococcus coralloides DSM 2259, which only produced DP2 and DP3 maltooligosaccharides from longer-chain substrates. This suggests that this enzyme is a maltogenic α-amylase [7].
Kinetics and Mechanism
Niallia circulans IgtZ is a retaining enzyme as Watanabe et al. [1] confirmed by polarimetry in 2006. The authors did not discuss the enzyme’s possible exo- or endo-acting mode of action.
Catalytic Residues
In silico studies have revealed a close phylogenetic relationship between GH families 119 and 57 [3, 4]. Through multiple sequence alignment (MSA) and superimposition of GH119 homology models and GH57 crystallographic structures, it has been demonstrated that GH119 sequences share the same five CSRs typical of GH57 [3]. The strictly conserved, catalytic residues of GH57, namely a strand β4 glutamate serving as nucleophile and a β7 aspartate serving as acid/base [8, 9] are located in CSR-3 and CSR-4, respectively. The equivalent residues in CocoGH119 (E225 and D369) have been found to be essential to catalysis by site-directed mutagenesis experiments resulting in complete abolishment of the enzyme’s activity [7]. Furthermore, homology models of GH119 sequences suggest that their putative catalytic domain may adopt a (β/α)7-barrel (i.e., an incomplete TIM-barrel) fold followed by a bundle of α-helices [3, 4]. These characteristics differentiate the amylolytic families GH57 and GH119 from the main α-amylase family, GH13, which adopts a (β/α)8-barrel fold (i.e., a classical TIM-barrel) and has a Asp-Glu-Asp catalytic triad, as also observed in GH70 and GH77, all of which are members of clan GH-H [2]. Based on these differences, GH119 and GH57 define clan GH-S [7].
Three-dimensional structures
No 3D structure of a GH119 protein has been experimentally established so far. However, domain prediction indicates that several GH119 proteins exhibit a multi-modular architecture consisting of an N-terminal, putatively-catalytic, main domain followed by a variable number of additional domains, including carbohydrate-binding modules (CBM) of families CBM20, CBM25 and CBM26, fibronectin type III (FN-III) and dockerin domains, among others [4, 7, 10]. A phylogenetic tree of the family annotated with predicted domain architecture (Fig. 2) shows that different branches exhibit distinctive auxiliary domain patterns [7]. Domain prediction for IgtZ suggests that this enzyme comprises an N-terminal, putative catalytic domain, followed by an FN-III, two CBM20 in tandem and a C-terminal CBM25 [4].
Figure 2. Phylogenetic tree representing 52 GH119 representative sequences [7]. Tree branches in the same clade share the same colour. Stars mark experimentally characterised sequences. The remaining annotations to each branch, from left to right, include: NCBI accession number, source organism name coloured by phylum and predicted sequence domain composition.
Family Firsts
- First stereochemistry determination
- The first evidence of a retaining mechanism within the family was presented by Watanabe and colleagues, who conducted a polarimetry study of the maltooligosaccharide products resulting from the hydrolysis of maltopentaosyl trehalose by IgtZ [1].
- First catalytic nucleophile identification
- Inferred to be a strictly conserved, strand β4 glutamate located in the CSR-3 of GH119 and GH57 MSAs [3, 4]. The equivalent residue in CocoGH119 (E225) is essential to catalysis as demonstrated through site-directed mutagenesis [7].
- First general acid/base residue identification
- As above, also inferred through alignment [3, 4] and confirmed by site-directed mutagenesis to be a fully conserved, β7 aspartate located in CSR-4 of the family (D369 in CocoGH119) [7].
- First 3-D structure
- Not yet determined.
References
- Watanabe H, Nishimoto T, Kubota M, Chaen H, and Fukuda S. (2006). Cloning, sequencing, and expression of the genes encoding an isocyclomaltooligosaccharide glucanotransferase and an alpha-amylase from a Bacillus circulans strain. Biosci Biotechnol Biochem. 2006;70(11):2690-702. DOI:10.1271/bbb.60294 |
-
Janeček Š and Svensson B (2022) How many α-amylase GH families are there in the CAZy database? Amylase. 6:1–10. DOI:10.1515/amylase-2022-0001
- Janeček S and Kuchtová A. (2012). In silico identification of catalytic residues and domain fold of the family GH119 sharing the catalytic machinery with the α-amylase family GH57. FEBS Lett. 2012;586(19):3360-6. DOI:10.1016/j.febslet.2012.07.020 |
-
Polaček A and Janeček Š (2023). Sequence-structural features and evolution of the α-amylase family GH119 revealed by the in silico analysis of its relatedness to the family GH57. Biologia. 78:1847–1860. DOI:10.1007/s11756-023-01349-y
- Janeček S and Blesák K. (2011). Sequence-structural features and evolutionary relationships of family GH57 α-amylases and their putative α-amylase-like homologues. Protein J. 2011;30(6):429-35. DOI:10.1007/s10930-011-9348-7 |
- Blesák K and Janeček S. (2012). Sequence fingerprints of enzyme specificities from the glycoside hydrolase family GH57. Extremophiles. 2012;16(3):497-506. DOI:10.1007/s00792-012-0449-9 |
- Vuillemin M, Moreno Prieto ES, Pilgaard B, Siebenhaar S, Holck J, Henrissat B, Bahieldin A, Hakeem KR, and Alghamdi KM. (2024). Biochemical exploration of family GH119 reveals a single α-amylase specificity and confirms shared catalytic machinery with GH57 enzymes. Int J Biol Macromol. 2024;262(Pt 2):129783. DOI:10.1016/j.ijbiomac.2024.129783 |
- Imamura H, Fushinobu S, Jeon BS, Wakagi T, and Matsuzawa H. (2001). Identification of the catalytic residue of Thermococcus litoralis 4-alpha-glucanotransferase through mechanism-based labeling. Biochemistry. 2001;40(41):12400-6. DOI:10.1021/bi011017c |
- Imamura H, Fushinobu S, Yamamoto M, Kumasaka T, Jeon BS, Wakagi T, and Matsuzawa H. (2003). Crystal structures of 4-alpha-glucanotransferase from Thermococcus litoralis and its complex with an inhibitor. J Biol Chem. 2003;278(21):19378-86. DOI:10.1074/jbc.M213134200 |
- Janeček Š, Mareček F, MacGregor EA, and Svensson B. (2019). Starch-binding domains as CBM families-history, occurrence, structure, function and evolution. Biotechnol Adv. 2019;37(8):107451. DOI:10.1016/j.biotechadv.2019.107451 |