CAZypedia needs your help! We have many unassigned GH, PL, CE, AA, GT, and CBM pages in need of Authors and Responsible Curators.
Scientists at all career stages, including students, are welcome to contribute to CAZypedia. Read more here, and in the 10th anniversary article in Glycobiology.
New to the CAZy classification? Read this first.
*
Consider attending the 15th Carbohydrate Bioengineering Meeting in Ghent, 5-8 May 2024.
Difference between revisions of "Phosphorylases"
| Line 3: | Line 3: | ||
---- | ---- | ||
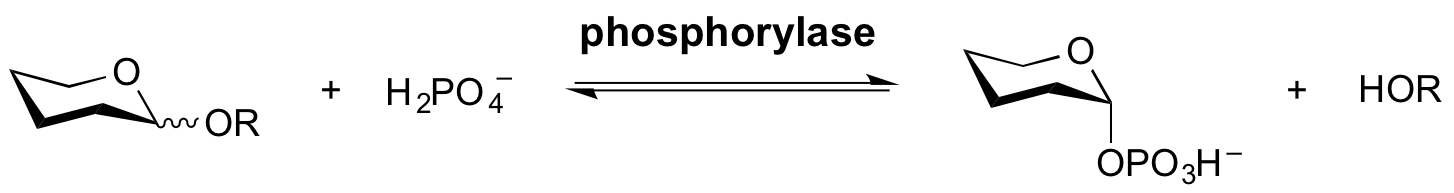
| − | Phosphorylases are enzymes that catalyze the phosphorolysis of the glycosidic bond. They are usually named using a combination of the ‘substrate name’ and ‘phosphorylase’. Phosphorolysis of a glycosidic bond can occur with retention or inversion of configuration and always occurs in an ''exo''-fashion leading to formation of a sugar-1-phosphate. | + | Phosphorylases are enzymes that catalyze the phosphorolysis of the glycosidic bond. They are usually named using a combination of the ‘substrate name’ and ‘phosphorylase’. |
| + | |||
| + | [[Image:Phosphorylase.png|centre]] | ||
| + | Phosphorolysis of a glycosidic bond can occur with retention or inversion of configuration and always occurs in an ''exo''-fashion leading to formation of a sugar-1-phosphate. | ||
| + | The energy content of the sugar-1-phosphate product means that the cleavage reaction is in a practical sense reversible and, in Nature, these enzymes may be used for either synthesis or cleavage of the glycosidic bond. As such there is a relatively fine distinction among sugar phosphorylases, [[glycoside hydrolase]]s and classical sugar nucleoside (di)phosphate dependent [[glycosyltransferases]]. In the last case the synthetic reaction is normally, but not always, irreversible because of the higher energy of a sugar nucleoside (di)phosphate. | ||
The classical example of a phosphorylase is glycogen phosphorylase.<sup>40</sup> This 'workhorse' enzyme catalyzes the cleavage of individual glucosyl residues from glycogen (up to five residues from a branchpoint), forming sequentially deglycosylated glycogen and glucose-1-phosphate. Glycogen phosphorylase has a complex mechanism that is not fully understood and requires pyridoxal phosphate (PLP) as a cofactor.<sup>10,41</sup> Glycogen phosphorylase is classified into the same sequence-related glycosyltransferase family as starch phosphorylases (GT35), which also require a PLP cofactor.<sup>41</sup> Most sugar phosphorylases act on glucosides and many cleave simple disaccharides such as sucrose, trehalose, cellobiose and maltose leading to glucose-1-phosphate and the other component sugar (glucose or fructose).<sup>39</sup> Other sugar phosphorylases are known that act on chitobiose, laminaribiose, 1,3-b-glucan and nucleosides.<sup>10,39</sup> Sequence and structural analysis of sugar phosphorylases reveal that many have sequences and structures (and likely mechanisms) similar to glycosyltransferases, whereas others have sequences and structures that more closely resemble glycoside hydrolases (GH13, GH65).<sup>42</sup> | The classical example of a phosphorylase is glycogen phosphorylase.<sup>40</sup> This 'workhorse' enzyme catalyzes the cleavage of individual glucosyl residues from glycogen (up to five residues from a branchpoint), forming sequentially deglycosylated glycogen and glucose-1-phosphate. Glycogen phosphorylase has a complex mechanism that is not fully understood and requires pyridoxal phosphate (PLP) as a cofactor.<sup>10,41</sup> Glycogen phosphorylase is classified into the same sequence-related glycosyltransferase family as starch phosphorylases (GT35), which also require a PLP cofactor.<sup>41</sup> Most sugar phosphorylases act on glucosides and many cleave simple disaccharides such as sucrose, trehalose, cellobiose and maltose leading to glucose-1-phosphate and the other component sugar (glucose or fructose).<sup>39</sup> Other sugar phosphorylases are known that act on chitobiose, laminaribiose, 1,3-b-glucan and nucleosides.<sup>10,39</sup> Sequence and structural analysis of sugar phosphorylases reveal that many have sequences and structures (and likely mechanisms) similar to glycosyltransferases, whereas others have sequences and structures that more closely resemble glycoside hydrolases (GH13, GH65).<sup>42</sup> | ||
Revision as of 04:20, 15 July 2009
- Author: Spencer Williams
- Responsible Editor: Spencer Williams
Phosphorylases are enzymes that catalyze the phosphorolysis of the glycosidic bond. They are usually named using a combination of the ‘substrate name’ and ‘phosphorylase’.
Phosphorolysis of a glycosidic bond can occur with retention or inversion of configuration and always occurs in an exo-fashion leading to formation of a sugar-1-phosphate. The energy content of the sugar-1-phosphate product means that the cleavage reaction is in a practical sense reversible and, in Nature, these enzymes may be used for either synthesis or cleavage of the glycosidic bond. As such there is a relatively fine distinction among sugar phosphorylases, glycoside hydrolases and classical sugar nucleoside (di)phosphate dependent glycosyltransferases. In the last case the synthetic reaction is normally, but not always, irreversible because of the higher energy of a sugar nucleoside (di)phosphate.
The classical example of a phosphorylase is glycogen phosphorylase.40 This 'workhorse' enzyme catalyzes the cleavage of individual glucosyl residues from glycogen (up to five residues from a branchpoint), forming sequentially deglycosylated glycogen and glucose-1-phosphate. Glycogen phosphorylase has a complex mechanism that is not fully understood and requires pyridoxal phosphate (PLP) as a cofactor.10,41 Glycogen phosphorylase is classified into the same sequence-related glycosyltransferase family as starch phosphorylases (GT35), which also require a PLP cofactor.41 Most sugar phosphorylases act on glucosides and many cleave simple disaccharides such as sucrose, trehalose, cellobiose and maltose leading to glucose-1-phosphate and the other component sugar (glucose or fructose).39 Other sugar phosphorylases are known that act on chitobiose, laminaribiose, 1,3-b-glucan and nucleosides.10,39 Sequence and structural analysis of sugar phosphorylases reveal that many have sequences and structures (and likely mechanisms) similar to glycosyltransferases, whereas others have sequences and structures that more closely resemble glycoside hydrolases (GH13, GH65).42