CAZypedia needs your help! We have many unassigned GH, PL, CE, AA, GT, and CBM pages in need of Authors and Responsible Curators.
Scientists at all career stages, including students, are welcome to contribute to CAZypedia. Read more here, and in the 10th anniversary article in Glycobiology.
New to the CAZy classification? Read this first.
*
Consider attending the 15th Carbohydrate Bioengineering Meeting in Ghent, 5-8 May 2024.
Difference between revisions of "Polysaccharide Lyase Family 9"
Harry Brumer (talk | contribs) m (Text replacement - "\^\^\^(.*)\^\^\^" to "$1") |
|||
| (16 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
<!-- RESPONSIBLE CURATORS: Please replace the {{UnderConstruction}} tag below with {{CuratorApproved}} when the page is ready for wider public consumption --> | <!-- RESPONSIBLE CURATORS: Please replace the {{UnderConstruction}} tag below with {{CuratorApproved}} when the page is ready for wider public consumption --> | ||
| − | {{ | + | {{CuratorApproved}} |
| − | * [[Author]]: | + | * [[Author]]: [[User:Ana Luis|Ana Luis]] |
| − | * [[Responsible Curator]]: | + | * [[Responsible Curator]]: [[User:Wade Abbott|Wade Abbott]] |
---- | ---- | ||
| Line 30: | Line 30: | ||
<!-- This is the end of the table --> | <!-- This is the end of the table --> | ||
== Substrate specificities == | == Substrate specificities == | ||
| − | Polysaccharide lyases of family 9 ([http://www.cazy.org/PL9.html CAZy]) | + | Polysaccharide lyases of family 9 ([http://www.cazy.org/PL9.html CAZy]) are active on pectins, a major plant cell wall polysaccharide. The main activity in characterized PL9 is pectate lyase. These enzymes cleave non-methylated α-(1-4)-linked D-galacturonic acid (homogalacturonan) by a β-elimination mechanism ([{{EClink}}4.2.2.2 EC 4.2.2.2]) <cite>Jenkins2004</cite>. Two PL9 endo-acting lyases have been shown to be active on rhamnogalacturonan-I ([{{EClink}}4.2.2.23 EC 4.2.2.23]) <cite>Luis2018</cite>. Additional activities include: exopolygalacturonic lyase ([{{EClink}}4.2.2.9 EC 4.2.2.9]) and thiopeptidoglycan lyase ([{{EClink}}4.2.2.- EC 4.2.2.-]) <cite>Brooks1990 Kondo2011</cite>. |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== Kinetics and Mechanism == | == Kinetics and Mechanism == | ||
| − | PL9 acts by an ''anti''-β-elimination mechanism generating a 4,5-unsaturated galacturonic acid product and a new reducing end. The elimination of C5 proton is base-catalyzed by lysine 237 <cite>Jenkins2004</cite>. Similar to [ | + | PL9 acts by an ''anti''-β-elimination mechanism generating a 4,5-unsaturated galacturonic acid product and a new reducing end. The elimination of C5 proton is base-catalyzed by lysine 237 <cite>Jenkins2004</cite>. Similar to the [[PL1]] family, a calcium ion interacts with the substrate carboxylate at +1 subsite promoting the C5 proton acidification. <cite>Jenkins2004 Seyedarabi2010</cite>. The characterization of the ''Bacteroides thetaiotaomicron'' rhamnogalacturonan lyase (BT4170) revealed an additional calcium ion also interacting with the substrate and playing a role in catalysis (Figure 1) <cite>Luis2018</cite>. |
== Catalytic Residues == | == Catalytic Residues == | ||
| − | The lysine 237 (K237) is the Brønstead base (responsible for the abstraction of the C5 proton from galacturonic acid at +1 subsite). The calcium coordination pocket is comprised of four aspartates (D209, D233, D234 and D237) <cite>Jenkins2004</cite>. | + | [[File:Active site PL9 1.png|thumb|300px|right|'''Figure 1.''' '''BT4170 ([{{PDBlink}}5OLR PDB ID 5OLR]) and Pel9A ([{{PDBlink}}1RU4 PDB ID 1RU4]) active site'''. Superimposed active residues of BT4170 (cyan) and Pel9A (green). The calcium ions are represented as spheres (gray). The first calcium is found in both structures. However, Ca<sup>2+</sup>_2 is only present in BT4170 struture.]] |
| − | + | In Pel9A the lysine 237 (K237) is the Brønstead base (responsible for the abstraction of the C5 proton from galacturonic acid at +1 subsite). The calcium coordination pocket is comprised of four aspartates (D209, D233, D234 and D237) <cite>Jenkins2004</cite>. These residues are essential in catalysis and invariant in PL9 family (Figure 1) <cite>Luis2018</cite>. In BT4170 rhamnogalacturonan lyase, the residues G212, D246 and D280 comprise a second calcium binding site that is not conserved in pectate lyases (Figure 1) <cite>Luis2018</cite>. | |
== Three-dimensional structures == | == Three-dimensional structures == | ||
| − | + | [[File:PL9.png|thumb|300px|right|'''Figure 2.''' '''Pel9A in complex with Ca<sup>2+</sup>''' ([{{PDBlink}}1RU4 PDB ID 1RU4]) '''A.''' Schematic representation of Pel9A parallel β-helix fold colour ramped from blue (N-terminal) to red (C-terminal). The active site is represented as sticks and highlighted inside the black box. The calcium is represented as sphere (gray) '''B.''' Blow up of the active site. The residues interacting with calcium and the proposed catalytic base (K237) are represented as stick in green and yellow, respectively.]] | |
| − | [[ | + | PL9 structure of ''Erwinia chrysanthemi'' (Pel9A) was solved at a resolution of 1.6 Å ([{{PDBlink}}1RU4 PDB ID 1RU4]) and displays a right-handed parallel β-helix fold (Figure 2A). The superhelical structure presents 10 complete coils and 3 β -sheets (PB1, PB2, PB3). A short α-helix at N-terminus caps the hydrophobic core of the parallel β -helix. The catalytic base K237 and calcium binding site are orientated in the structure cleft (Figure 2B) <cite>Jenkins2004</cite>. The structure of the rhamnogalacturonan lyase (BT4170) in complex with the enzyme product showed that apart from the catalytic apparatus, there is little conservation of substrate binding residues between this enzyme and the pectate lyase Pel9A <cite>Luis2018</cite>. |
| − | |||
| − | File:PL9.png|thumb|300px|right|'''Figure | ||
| − | |||
| − | |||
| − | PL9 structure of ''Erwinia chrysanthemi'' (Pel9A) was solved at a resolution of 1.6 Å ([ | ||
== Family Firsts == | == Family Firsts == | ||
| Line 61: | Line 51: | ||
<biblio> | <biblio> | ||
#Jenkins2004 pmid=14670977 | #Jenkins2004 pmid=14670977 | ||
| − | # | + | #Luis2018 pmid=29255254 |
#Kondo2011 pmid=21095202 | #Kondo2011 pmid=21095202 | ||
#Seyedarabi2010 pmid=20000851 | #Seyedarabi2010 pmid=20000851 | ||
Latest revision as of 14:19, 18 December 2021
This page has been approved by the Responsible Curator as essentially complete. CAZypedia is a living document, so further improvement of this page is still possible. If you would like to suggest an addition or correction, please contact the page's Responsible Curator directly by e-mail.
| Polysaccharide Lyase Family PL9 | |
| 3D Structure | β-helix |
| Mechanism | β-elimination |
| Charge neutraliser | calcium |
| Active site residues | known |
| CAZy DB link | |
| http://www.cazy.org/PL9.html | |
Substrate specificities
Polysaccharide lyases of family 9 (CAZy) are active on pectins, a major plant cell wall polysaccharide. The main activity in characterized PL9 is pectate lyase. These enzymes cleave non-methylated α-(1-4)-linked D-galacturonic acid (homogalacturonan) by a β-elimination mechanism (EC 4.2.2.2) [1]. Two PL9 endo-acting lyases have been shown to be active on rhamnogalacturonan-I (EC 4.2.2.23) [2]. Additional activities include: exopolygalacturonic lyase (EC 4.2.2.9) and thiopeptidoglycan lyase (EC 4.2.2.-) [3, 4].
Kinetics and Mechanism
PL9 acts by an anti-β-elimination mechanism generating a 4,5-unsaturated galacturonic acid product and a new reducing end. The elimination of C5 proton is base-catalyzed by lysine 237 [1]. Similar to the PL1 family, a calcium ion interacts with the substrate carboxylate at +1 subsite promoting the C5 proton acidification. [1, 5]. The characterization of the Bacteroides thetaiotaomicron rhamnogalacturonan lyase (BT4170) revealed an additional calcium ion also interacting with the substrate and playing a role in catalysis (Figure 1) [2].
Catalytic Residues
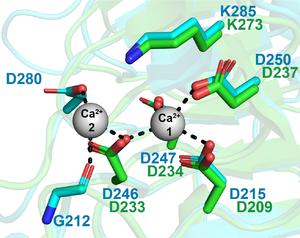
In Pel9A the lysine 237 (K237) is the Brønstead base (responsible for the abstraction of the C5 proton from galacturonic acid at +1 subsite). The calcium coordination pocket is comprised of four aspartates (D209, D233, D234 and D237) [1]. These residues are essential in catalysis and invariant in PL9 family (Figure 1) [2]. In BT4170 rhamnogalacturonan lyase, the residues G212, D246 and D280 comprise a second calcium binding site that is not conserved in pectate lyases (Figure 1) [2].
Three-dimensional structures
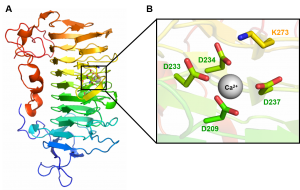
PL9 structure of Erwinia chrysanthemi (Pel9A) was solved at a resolution of 1.6 Å (PDB ID 1RU4) and displays a right-handed parallel β-helix fold (Figure 2A). The superhelical structure presents 10 complete coils and 3 β -sheets (PB1, PB2, PB3). A short α-helix at N-terminus caps the hydrophobic core of the parallel β -helix. The catalytic base K237 and calcium binding site are orientated in the structure cleft (Figure 2B) [1]. The structure of the rhamnogalacturonan lyase (BT4170) in complex with the enzyme product showed that apart from the catalytic apparatus, there is little conservation of substrate binding residues between this enzyme and the pectate lyase Pel9A [2].
Family Firsts
- First description of catalytic activity
- PelX from Erwinia chrysanthemi [3].
- First catalytic base identification
- Pel9A from Erwinia chrysanthemi [1].
- First catalytic divalent cation identification
- Pel9A from Erwinia chrysanthemi [1].
- First 3-D structure
- Pel9A from Erwinia chrysanthemi [1].
References
- Jenkins J, Shevchik VE, Hugouvieux-Cotte-Pattat N, and Pickersgill RW. (2004). The crystal structure of pectate lyase Pel9A from Erwinia chrysanthemi. J Biol Chem. 2004;279(10):9139-45. DOI:10.1074/jbc.M311390200 |
- Luis AS, Briggs J, Zhang X, Farnell B, Ndeh D, Labourel A, Baslé A, Cartmell A, Terrapon N, Stott K, Lowe EC, McLean R, Shearer K, Schückel J, Venditto I, Ralet MC, Henrissat B, Martens EC, Mosimann SC, Abbott DW, and Gilbert HJ. (2018). Dietary pectic glycans are degraded by coordinated enzyme pathways in human colonic Bacteroides. Nat Microbiol. 2018;3(2):210-219. DOI:10.1038/s41564-017-0079-1 |
- Brooks AD, He SY, Gold S, Keen NT, Collmer A, and Hutcheson SW. (1990). Molecular cloning of the structural gene for exopolygalacturonate lyase from Erwinia chrysanthemi EC16 and characterization of the enzyme product. J Bacteriol. 1990;172(12):6950-8. DOI:10.1128/jb.172.12.6950-6958.1990 |
- Kondo K, Takeda M, Ejima W, Kawasaki Y, Umezu T, Yamada M, Koizumi J, Mashima T, and Katahira M. (2011). Study of a novel glycoconjugate, thiopeptidoglycan, and a novel polysaccharide lyase, thiopeptidoglycan lyase. Int J Biol Macromol. 2011;48(2):256-62. DOI:10.1016/j.ijbiomac.2010.11.009 |
- Seyedarabi A, To TT, Ali S, Hussain S, Fries M, Madsen R, Clausen MH, Teixteira S, Brocklehurst K, and Pickersgill RW. (2010). Structural insights into substrate specificity and the anti beta-elimination mechanism of pectate lyase. Biochemistry. 2010;49(3):539-46. DOI:10.1021/bi901503g |