CAZypedia needs your help! We have many unassigned GH, PL, CE, AA, GT, and CBM pages in need of Authors and Responsible Curators.
Scientists at all career stages, including students, are welcome to contribute to CAZypedia. Read more here, and in the 10th anniversary article in Glycobiology.
New to the CAZy classification? Read this first.
*
Consider attending the 15th Carbohydrate Bioengineering Meeting in Ghent, 5-8 May 2024.
Difference between revisions of "Reaction intermediate"
Harry Brumer (talk | contribs) m |
|||
| Line 7: | Line 7: | ||
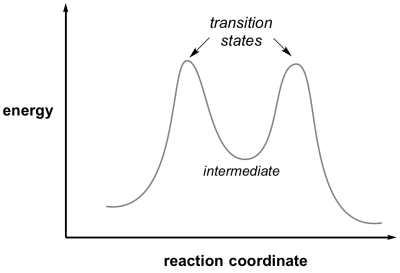
An '''intermediate''' in a reaction is a species formed that has a real lifetime, best defined as being greater than the time for a bond vibration (approx 10<sup>-12 </sup>sec). This will correspond to a minimum in the reaction coordinate diagram located between the reactant and product. Depending on their lifetimes and the tools available, intermediates can sometimes be trapped and observed by physical and chemical techniques. | An '''intermediate''' in a reaction is a species formed that has a real lifetime, best defined as being greater than the time for a bond vibration (approx 10<sup>-12 </sup>sec). This will correspond to a minimum in the reaction coordinate diagram located between the reactant and product. Depending on their lifetimes and the tools available, intermediates can sometimes be trapped and observed by physical and chemical techniques. | ||
| − | [[Image:ReactionCoordinate.png| | + | [[Image:ReactionCoordinate.png|center|400px]] |
[[Category:Definitions and explanations]] | [[Category:Definitions and explanations]] | ||
Revision as of 03:08, 1 May 2013
This page has been approved by the Responsible Curator as essentially complete. CAZypedia is a living document, so further improvement of this page is still possible. If you would like to suggest an addition or correction, please contact the page's Responsible Curator directly by e-mail.
- Author: Stephen Withers
- Responsible Curator: Spencer Williams
An intermediate in a reaction is a species formed that has a real lifetime, best defined as being greater than the time for a bond vibration (approx 10-12 sec). This will correspond to a minimum in the reaction coordinate diagram located between the reactant and product. Depending on their lifetimes and the tools available, intermediates can sometimes be trapped and observed by physical and chemical techniques.