CAZypedia celebrates the life of Senior Curator Emeritus Harry Gilbert, a true giant in the field, who passed away in September 2025.
CAZypedia needs your help!
We have many unassigned pages in need of Authors and Responsible Curators. See a page that's out-of-date and just needs a touch-up? - You are also welcome to become a CAZypedian. Here's how.
Scientists at all career stages, including students, are welcome to contribute.
Learn more about CAZypedia's misson here and in this article. Totally new to the CAZy classification? Read this first.
Polysaccharide Lyase Family 7
This page is currently under construction. This means that the Responsible Curator has deemed that the page's content is not quite up to CAZypedia's standards for full public consumption. All information should be considered to be under revision and may be subject to major changes.
- Authors: ^^^Nadine Gerlach^^^
- Responsible Curator: ^^^Jan-Hendrik Hehemann^^^
| Polysaccharide Lyase Family PL7 | |
| 3D Structure | β jelly roll |
| Mechanism | β-elimination |
| Active site residues | R, Q, H, 2xY |
| CAZy DB link | |
| https://www.cazy.org/PL7.html | |
Mechanism
Alginate lyases (Alys) of all families, PL5-7, PL14-15, PL17-18, catalyze the depolymerization of alginate in three steps: (I) removal of the negative charge on the carboxylate anion, (II) general base-catalyzed abstraction of the proton on the C5 and (III) β-elimination of the 4-O-glycosidic bond [2]. Most PL7s are endo-active, i.e. acting within a poly- or oligosaccharide and releasing smaller alginate fragments. Some PL7 are exo-acting cleaving a monosaccharide from the non-reducing end of the polymer [3]. In both modes of action, a new non-reducing end with a 4-deoxy-L-erythro-hex-4-en pyranosyl uronate residue (Δ) is formed.
Some marine PL7 alginate lyases require a calcium cation for substrate recognition and binding [3]. Ca2+ is weakening the ionic interactions between substrate (polyanion) and PL7 (polycation) by reducing the surface density of the alginate, charge and therefore increasing the enzyme activity [4]. However, there have also been reports where mono- or divalent (metal) cations did not increase or even decreased enzymatic activity [5, 6]. Therefore, it is not clear if Ca2+ is required for recognition and / or binding. However, one could speculate if cations like Ca2+ are creating a more accessible form of alginate. In the presence of divalent cations, the confirmation of alginate is changed due to cross linkages with opposition guluronate residues forming a so-called egg box model [7]. Thereby, the 3D confirmation of alginate is changed, forming hydrogels which might be an easier target in the marine environment as its dissolved form.
Kinetics and catalytic residues
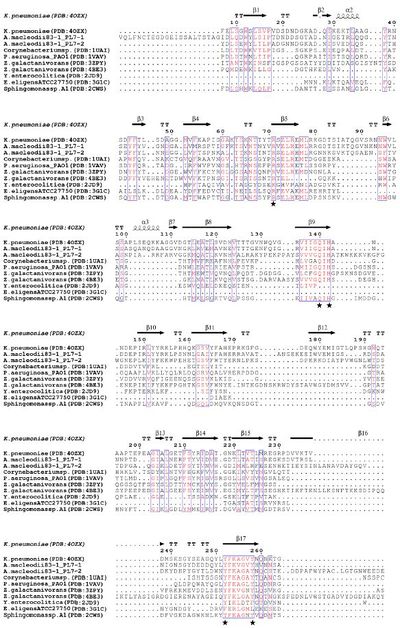
Several structural and biochemical analyses of wild type and mutated PL7s revealed five residues forming the active site: arginine (R), glutamine (Q), histidine (H), tyrosine (Y) [8, 9, 10], which are present in three highly conserved regions: R*ELR*ML, VIIGQ(I/V)H, YFKAG*Y*Q respectively (Figure 1) [11]. It has been proposed that in PL7 ALY-1 from Corynebacterium sp. Q117+Y195 interact near the reaction site of alginate to maintain proper orientation of the substrate, R72 interacts with alginate due to the formation of salt bridges with the carboxyl groups at the C5 and H119 acts as a base to deprotonate [12]. However, there can also be additional charged residues at the active site, which promote substrate recognition and binding [3]. Such residues can be found in the N-terminal R*ELREML and VIIGQIH regions. Both highly conserved regions are mainly characterized by hydrophobic amino acids (especially aromatic amino acids) such as leucine, tryptophan and methionine as well as residues with planar polar side chains (especially amino acids with charged side chains) such as arginine, glutamic acid, glutamine (Figure 2). These residues have been suggested to be substrate-binding molecules [11].
Substrate specificities
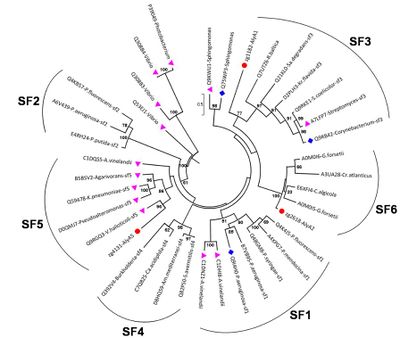
Polysaccharide lyase family 7 (PL7) contains five subfamilies (SF) based on their sequence similarities [13], plus a so far uncharacterized sixth subfamily, which consist only of marine representatives of the Flavobacteriaceae (Figure 2) [3]. The substrate specificity depends on the source of alginate, i.e. derived from brown seaweed or mucoid bacteria Pseudomonas spp. and Azotobacter vinelandii, as well as geographical and seasonal parameters. Alginate is a heteropolysaccharide, consisting of β-D-mannuronate (M) and α-L-guluronate (G). These monosaccharides can occur in homogenous and heterogenous blocks. In addition, bacterial alginate is often acetylated at the C2 and / or C3 of mannuronate, thereby shielding the substrate from degradation. Hence, PL7s can be mannuronate (EC 4.2.2.3), guluronate (EC 4.2.2.11) or mixed link (EC 4.2.2.-) specific lyases. Despite the preference for M- or G-enriched blocks, most PL7 also have a low to moderate activity for the other building block [3, 6, 14]. PolyG specific PL7 have been found in the SF3 and SF5 [3] with QIH in the second highly conserved region, while polyM specific PL7s are characterized by QVH [15, 16].
Three-dimensional structures
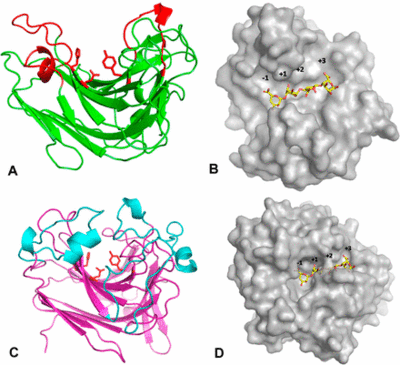
PL7 displays a jelly roll fold, which is also found in PL14 as well as in glycoside hydrolases of family GH16. Zobellia galactanivorans DsijT possess, among others, two PL7 with different modes of activity [3]. AlyA1 is an endo-acting PL7 belonging to SF3 with a wide open cleft harboring the active site (Figure 3A, B), whereas AlyA5 belongs to SF5 and is exo-active which active site is closed by three loops forming a small pocket (Figure 3C, D). The highly conserved 9-amino-acid-block YFKAGVY*Q (where * is a variable residue) at the C-terminus of PL7s (Figure 2) has also been found for an extracellular pectate lyase (PL1, PDB: 1AIR) in E. chrysanthemi. Alginate and pectate/pectin lyases share the β-elimination mechanism, the recognition of substrates of a similar structure, Ca2+-binding site and primary sequence similarity, indicating that they probably possess a similar core structural fold probably important to maintain a stable 3D-confirmation [11]. Several alginate lyases have been reported to be multimodular enzymes with putative non-catalytic, carbohydrate-binding modules (CBMs). The first biochemically characterized CBM of an endo PL7 was a N-terminal CBM13 from Agarivorans sp. L11, which increased its substrate binding and therefore catalytic efficiency, influenced substrate specificity, the product profile and thermo stability [18]. Contradictory observations regarding catalytic efficiency and substrate specificity were made for an endo PL7 from Vibrio splendidus OU02 DNA with an N-terminal CBM32 linked by a unique alpha-helix linker. The CBM and linker were proposed to serve as a "pivot point" somehow just pushing the product profile towards trisaccharides [19]. The PL7 from Persicobacter sp. CCB-QB2 consists of three domains - a N-terminal CBM16 with unknown function, a CBM32 and the C-terminal PL7 [14]. Overall the role of CBMs from different families as part of PL7s still remains unclear. Another common structural feature found in PLs are flexible loops facing above the active site [20], which have been demonstrated to influence substrate recognition, binding [3, 21] and potentially specificity [22].
Gene transfer of Alys among different habitats
Alginate degrading organisms often posse specified gene clusters for glycan utilization which contain, among other proteins such as transporters, several endo- and exo-acting Alys of different families. These gene clusters are called polysaccharide utilization loci (PULs) for Bacteriodetes [23] or alginolytic operons in case a SusCD pair transporter is missing or replaced by a different transporter system. It has been shown that these gene clusters can be transferred horizontally from one organism to another and thereby even cross different environmental habitats [24, 25]. The first alginate utilization system (AUS) was found in Zobellia galactinovorans which contains two clusters harboring five out of seven Alys (3x PL7). Those operons originated from an ancestral marine Flavobacterium and were independently transferred to marine Proteobacteria and Japanese gut Bacteriodetes by lateral gene transfer (LGT) [26].
A more detailed studied on horizontal gene transfer of PL7 between marine, ecophysiological different Vibrionaceae isolates revealed rapid adaptation of closely related but speciated bacterial populations, resulting into a fine scale of resource partitioning. The exchange of PL7 between marine microbes drove the evolution of polysaccharide degrading pathways, which might have led to three ecotypes – the pioneers, which degrade the polymer into oligomers, the harvester (intermediate of the other two types) and the scavenger, which can only utilize very small oligos created by the pioneers [27].
Family Firsts
- First catalytic endo-activity
- polyM PL7 from Photobacterium ATCC 433367 [28], polyG PL7 from Klebsiella pneumoniae subbsp. aerogenes [29]
- First catalytic exo-activity
- AlyA5 from Zobellia galactanivorans DsijT [3]
- First 3-D apo-structure
- PA1167 from Pseudomonas aeruginosa [30]
- First 3-D holo-structure
- A1-II from Sphingomons sp. A1 [31]
- First characterised CBM
- AlyL2 containing a N-terminal CBM13 from Agarivorans sp. L11 [18]
References
- Robert X and Gouet P. (2014). Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 2014;42(Web Server issue):W320-4. DOI:10.1093/nar/gku316 |
-
Gacesa P. (1987) Alginate‐modifying enzymes: A proposed unified mechanism of action for the lyases and epimerases. FEBS Letters, 212, 1873-3468. DOI:10.1016/0014-5793(87)81344-3
- Thomas F, Lundqvist LC, Jam M, Jeudy A, Barbeyron T, Sandström C, Michel G, and Czjzek M. (2013). Comparative characterization of two marine alginate lyases from Zobellia galactanivorans reveals distinct modes of action and exquisite adaptation to their natural substrate. J Biol Chem. 2013;288(32):23021-37. DOI:10.1074/jbc.M113.467217 |
- Favorov VV, Vozhova EI, Denisenko VA, and Elyakova LA. (1979). A study of the reaction catalysed by alginate lyase VI from the sea mollusc, Littorina sp. Biochim Biophys Acta. 1979;569(2):259-66. DOI:10.1016/0005-2744(79)90061-5 |
- Jagtap SS, Hehemann JH, Polz MF, Lee JK, and Zhao H. (2014). Comparative biochemical characterization of three exolytic oligoalginate lyases from Vibrio splendidus reveals complementary substrate scope, temperature, and pH adaptations. Appl Environ Microbiol. 2014;80(14):4207-14. DOI:10.1128/AEM.01285-14 |
- Badur AH, Jagtap SS, Yalamanchili G, Lee JK, Zhao H, and Rao CV. (2015). Alginate lyases from alginate-degrading Vibrio splendidus 12B01 are endolytic. Appl Environ Microbiol. 2015;81(5):1865-73. DOI:10.1128/AEM.03460-14 |
-
Grant, G., Morris, E., Rees, D., Smith, P., and Thom, D.Biological interactions between polysaccharides and diva-lent cations: the egg-box model. FEBS Lett32,195, 1973.
- Preston LA, Wong TY, Bender CL, and Schiller NL. (2000). Characterization of alginate lyase from Pseudomonas syringae pv. syringae. J Bacteriol. 2000;182(21):6268-71. DOI:10.1128/JB.182.21.6268-6271.2000 |
- Yamasaki M, Ogura K, Hashimoto W, Mikami B, and Murata K. (2005). A structural basis for depolymerization of alginate by polysaccharide lyase family-7. J Mol Biol. 2005;352(1):11-21. DOI:10.1016/j.jmb.2005.06.075 |
- Wong TY, Preston LA, and Schiller NL. (2000). ALGINATE LYASE: review of major sources and enzyme characteristics, structure-function analysis, biological roles, and applications. Annu Rev Microbiol. 2000;54:289-340. DOI:10.1146/annurev.micro.54.1.289 |
- Osawa T, Matsubara Y, Muramatsu T, Kimura M, and Kakuta Y. (2005). Crystal structure of the alginate (poly alpha-l-guluronate) lyase from Corynebacterium sp. at 1.2 A resolution. J Mol Biol. 2005;345(5):1111-8. DOI:10.1016/j.jmb.2004.10.081 |
- Lombard V, Bernard T, Rancurel C, Brumer H, Coutinho PM, and Henrissat B. (2010). A hierarchical classification of polysaccharide lyases for glycogenomics. Biochem J. 2010;432(3):437-44. DOI:10.1042/BJ20101185 |
- Sim PF, Furusawa G, and Teh AH. (2017). Functional and Structural Studies of a Multidomain Alginate Lyase from Persicobacter sp. CCB-QB2. Sci Rep. 2017;7(1):13656. DOI:10.1038/s41598-017-13288-1 |
- Zhu B and Yin H. (2015). Alginate lyase: Review of major sources and classification, properties, structure-function analysis and applications. Bioengineered. 2015;6(3):125-31. DOI:10.1080/21655979.2015.1030543 |
- Deng S, Ye J, Xu Q, and Zhang H. (2014). Structural and functional studies on three alginate lyases from Vibrio alginolyticus. Protein Pept Lett. 2014;21(2):179-87. DOI:10.2174/09298665113206660094 |
-
Delano WL. (2002) Pymol: An open-source molecular graphics tool. CCP4 Newsletter On Protein Crystallography, 40, 82-92. Direct link.
- Li S, Yang X, Bao M, Wu Y, Yu W, and Han F. (2015). Family 13 carbohydrate-binding module of alginate lyase from Agarivorans sp. L11 enhances its catalytic efficiency and thermostability, and alters its substrate preference and product distribution. FEMS Microbiol Lett. 2015;362(10). DOI:10.1093/femsle/fnv054 |
- Lyu Q, Zhang K, Zhu Q, Li Z, Liu Y, Fitzek E, Yohe T, Zhao L, Li W, Liu T, Yin Y, and Liu W. (2018). Structural and biochemical characterization of a multidomain alginate lyase reveals a novel role of CBM32 in CAZymes. Biochim Biophys Acta Gen Subj. 2018;1862(9):1862-1869. DOI:10.1016/j.bbagen.2018.05.024 |
- Xu F, Wang P, Zhang YZ, and Chen XL. (2018). Diversity of Three-Dimensional Structures and Catalytic Mechanisms of Alginate Lyases. Appl Environ Microbiol. 2018;84(3). DOI:10.1128/AEM.02040-17 |
- Ogura K, Yamasaki M, Mikami B, Hashimoto W, and Murata K. (2008). Substrate recognition by family 7 alginate lyase from Sphingomonas sp. A1. J Mol Biol. 2008;380(2):373-85. DOI:10.1016/j.jmb.2008.05.008 |
- Qin HM , Miyakawa T , Inoue A , Nishiyama R , Nakamura A , Asano A , Ojima T , and Tanokura M . (2018). Structural basis for controlling the enzymatic properties of polymannuronate preferred alginate lyase FlAlyA from the PL-7 family. Chem Commun (Camb). 2018;54(5):555-558. DOI:10.1039/c7cc06523j |
- Bjursell MK, Martens EC, and Gordon JI. (2006). Functional genomic and metabolic studies of the adaptations of a prominent adult human gut symbiont, Bacteroides thetaiotaomicron, to the suckling period. J Biol Chem. 2006;281(47):36269-79. DOI:10.1074/jbc.M606509200 |
- Hehemann JH, Correc G, Barbeyron T, Helbert W, Czjzek M, and Michel G. (2010). Transfer of carbohydrate-active enzymes from marine bacteria to Japanese gut microbiota. Nature. 2010;464(7290):908-12. DOI:10.1038/nature08937 |
- Mathieu S, Touvrey-Loiodice M, Poulet L, Drouillard S, Vincentelli R, Henrissat B, Skjåk-Bræk G, and Helbert W. (2018). Ancient acquisition of "alginate utilization loci" by human gut microbiota. Sci Rep. 2018;8(1):8075. DOI:10.1038/s41598-018-26104-1 |
- Thomas F, Barbeyron T, Tonon T, Génicot S, Czjzek M, and Michel G. (2012). Characterization of the first alginolytic operons in a marine bacterium: from their emergence in marine Flavobacteriia to their independent transfers to marine Proteobacteria and human gut Bacteroides. Environ Microbiol. 2012;14(9):2379-94. DOI:10.1111/j.1462-2920.2012.02751.x |
- Hehemann JH, Arevalo P, Datta MS, Yu X, Corzett CH, Henschel A, Preheim SP, Timberlake S, Alm EJ, and Polz MF. (2016). Adaptive radiation by waves of gene transfer leads to fine-scale resource partitioning in marine microbes. Nat Commun. 2016;7:12860. DOI:10.1038/ncomms12860 |
- Malissard M, Duez C, Guinand M, Vacheron MJ, Michel G, Marty N, Joris B, Thamm I, and Ghuysen JM. (1993). Sequence of a gene encoding a (poly ManA) alginate lyase active on Pseudomonas aeruginosa alginate. FEMS Microbiol Lett. 1993;110(1):101-6. DOI:10.1111/j.1574-6968.1993.tb06302.x |
- Baron AJ, Wong TY, Hicks SJ, Gacesa P, Willcock D, and McPherson MJ. (1994). Alginate lyase from Klebsiella pneumoniae, subsp. aerogenes: gene cloning, sequence analysis and high-level production in Escherichia coli. Gene. 1994;143(1):61-6. DOI:10.1016/0378-1119(94)90605-x |
- Yamasaki M, Moriwaki S, Miyake O, Hashimoto W, Murata K, and Mikami B. (2004). Structure and function of a hypothetical Pseudomonas aeruginosa protein PA1167 classified into family PL-7: a novel alginate lyase with a beta-sandwich fold. J Biol Chem. 2004;279(30):31863-72. DOI:10.1074/jbc.M402466200 |
- Lombard V, Golaconda Ramulu H, Drula E, Coutinho PM, and Henrissat B. (2014). The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014;42(Database issue):D490-5. DOI:10.1093/nar/gkt1178 |