CAZypedia needs your help! We have many unassigned GH, PL, CE, AA, GT, and CBM pages in need of Authors and Responsible Curators.
Scientists at all career stages, including students, are welcome to contribute to CAZypedia. Read more here, and in the 10th anniversary article in Glycobiology.
New to the CAZy classification? Read this first.
*
Consider attending the 15th Carbohydrate Bioengineering Meeting in Ghent, 5-8 May 2024.
Difference between revisions of "Carbohydrate Binding Module Family 44"
| (26 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
<!-- RESPONSIBLE CURATORS: Please replace the {{UnderConstruction}} tag below with {{CuratorApproved}} when the page is ready for wider public consumption --> | <!-- RESPONSIBLE CURATORS: Please replace the {{UnderConstruction}} tag below with {{CuratorApproved}} when the page is ready for wider public consumption --> | ||
| − | {{ | + | {{CuratorApproved}} |
* [[Author]]: [[User:Marie-Katherin Zuehlke|Marie-Katherin Zühlke]] | * [[Author]]: [[User:Marie-Katherin Zuehlke|Marie-Katherin Zühlke]] | ||
* [[Responsible Curator]]: [[User:Elizabeth Ficko-Blean|Elizabeth Ficko-Blean]] | * [[Responsible Curator]]: [[User:Elizabeth Ficko-Blean|Elizabeth Ficko-Blean]] | ||
| Line 12: | Line 12: | ||
|{{Hl2}} colspan="2" align="center" |'''CAZy DB link''' | |{{Hl2}} colspan="2" align="center" |'''CAZy DB link''' | ||
|- | |- | ||
| − | | colspan="2" |{{CAZyDBlink}} | + | | colspan="2" |{{CAZyDBlink}}CBM44.html |
|} | |} | ||
</div> | </div> | ||
| Line 18: | Line 18: | ||
== Ligand specificities == | == Ligand specificities == | ||
| − | + | CBM44, from the CtCel9D-Cel44A enzyme produced by ''Acetivibrio thermocellus'' (formerly ''Clostridium thermocellum''), targets ß-1,4-polymers such as xyloglucan and cellulose (hydroxyethylcellulose and Avicel), mixed linkage ß-1,3/ß-1,4- glucans (lichenan and barley) or glucomannan (konjac) <cite>Najmudin2006</cite> and is classified as a [[Carbohydrate-binding_modules#Types|type B]] CBM. Affinity for xylan was very low and binding to laminarin, curdlan, pullulan, pustulan, galactomannan or galactan was negative. Isothermal titration calorimetry (ITC) revealed highest affinity for xyloglucan as a polysaccharide (~81.6 x 10<sup>4</sup> M<sup>-1</sup>), which was comparable to the affinity for cellohexaose as an oligosaccharide (~72.8 x 10<sup>4</sup> M<sup>-1</sup>). For other cello-oligosaccharides, this affinity decreased with decreasing chain length, while no binding was detected for cellotriose <cite>Najmudin2006</cite>. | |
| − | + | == Structural Features == | |
| + | Like many CBMs, CBM44 exhibits a typical ß-sandwich fold: two antiparallel ß-sheets form a concave and a convex surface. The concave surface forms a deep hydrophobic ligand-binding cleft that is estimated to accommodate up to five glucose residues (~24 Å). Here, three tryptophans (W189, W194 and W198) act as key residues to mediate ligand binding (Figure 1), as confirmed by affinity gel electrophoresis (AGE) and ITC analyses of specific mutants <cite>Najmudin2006</cite>. The orientation of the tryptophans corresponds to the slightly twisted conformation of cello-oligosaccharides in solution <cite>Sugiyama2000</cite>. | ||
| − | + | [[File:pkd-cbm44.png|thumb|200px|right|'''Figure 1.''' Three-dimensional structure of PKD-CBM44 as two domains of the multimodular cellulase ''Ct''Cel9D-Cel44A from ''Acetivibrio thermocellus'' ([{{PDBlink}}2c26 PDB 2c26]) <cite>Najmudin2006</cite>. The PKD domain is shown in orange, CBM44 in purple. The tryptophans involved in ligand recognition are highlighted. The calcium atoms of both modules are shown as red spheres.]] | |
| − | |||
| − | |||
| − | |||
| − | |||
== Functionalities == | == Functionalities == | ||
| − | + | Carbohydrate binding and targeting were described as functions of CBM44. While carbohydrate binding was confirmed by AGE and ITC, the [[Carbohydrate-binding modules|targeting effect]] was analyzed by the ability of CBM44 to maintain enzyme activity of [[GH44]] (Cel44A) exposed to a substrate mixture <cite>Najmudin2006</cite>. The ensemble of a [[GH44]] and a CBM44 was found in the multimodular cellulase CtCel9D-Cel44A from ''Acetivibrio thermocellus'' and ligand spectra of both modules are consistent <cite>Najmudin2006</cite>. In the targeting experiment, the fused [[GH44]]-CBM44 construct was compared to the truncated [[GH44]]. In separate xyloglucan or carboxymethylcellulose incubations, the activity of GH44-CBM44 was comparable to that of [[GH44]] alone. In a next step, substrate mixtures were used. These mixtures also contained laminarin and pustulan, which were not degraded. While [[GH44]] activity was decreased, activity was restored by [[GH44]]-CBM44. Thus, given the complex carbohydrates present in plant cell walls, CBM44 may guide the enzyme to its target sugars. Such effects have also been achieved in synthetic CBM44-enzyme chimeras, e.g. [[GH12]] or [[GH28]] <cite>#Furtado2015 #Carli2022</cite>. | |
| − | |||
| − | |||
| − | |||
== Family Firsts == | == Family Firsts == | ||
| − | ;First Identified | + | ;First Identified: CBM44 was first described as an unknown C-terminal domain in the multimodular cellulase ''Ct''Cel9D-Cel44A in ''Acetivibrio thermocellus'', formerly designated as CelJ and as ''Clostridium thermocellum'' <cite>#Ahsan1996 #Arai2003</cite>. The protein also features two catalytic domains ([[GH44]] and [[GH9]]), a [[CBM30]] and an internal dockerin to target the protein to the cellulosome. The C-terminal region was then identified as having a PKD module as well as a novel CBM binding ß-1,4-glucans, hereby founding the family 44 <cite>Najmudin2006</cite>. |
| − | : | + | ;First Structural Characterization: The same CBM44, together with the preceding PKD module, represents the first structurally characterized candidate of this family ([{{PDBlink}}2c26 PDB 2c26]) <cite>Najmudin2006</cite>. |
| − | ;First Structural Characterization | ||
| − | : | ||
== References == | == References == | ||
<biblio> | <biblio> | ||
| − | # | + | #Najmudin2006 pmid=16314409 |
| − | # | + | #Sugiyama2000 Sugiyama H, Hisamichi K, Usui T, Sakai K, Ishiyama J (2000). ''A study of the conformation of beta-1,4-linked glucose oligomers, cellobiose to cellohexaose, in solution.'' J Mol Struct. 2000;'''556'''(1-3):173-7. [https://doi.org/10.1016/S0022-2860(00)00630-X DOI: 10.1016/S0022-2860(00)00630-X]. |
| − | # | + | #Furtado2015 pmid=25605422 |
| − | # | + | #Carli2022 Carli S, Meleiro LP, Salgado JCS, Ward RJ (2022). ''Synthetic carbohydrate-binding module-endogalacturonase chimeras increase catalytic efficiency and saccharification of lignocellulose residues''. Biomass Conv Bioref. 2022. [https://doi.org/10.1007/s13399-022-02716-6 DOI: 10.1007/s13399-022-02716-6]. |
| − | + | #Ahsan1996 pmid=8824619 | |
| − | # | + | #Arai2003 pmid=12511497 |
| − | # | ||
</biblio> | </biblio> | ||
[[Category:Carbohydrate Binding Module Families|CBM044]] <!-- ATTENTION: Make sure to replace "nnn" with a three digit family number, e.g. "032" or "105" etc., for proper sorting of the page by family number. --> | [[Category:Carbohydrate Binding Module Families|CBM044]] <!-- ATTENTION: Make sure to replace "nnn" with a three digit family number, e.g. "032" or "105" etc., for proper sorting of the page by family number. --> | ||
Latest revision as of 01:25, 11 January 2023
This page has been approved by the Responsible Curator as essentially complete. CAZypedia is a living document, so further improvement of this page is still possible. If you would like to suggest an addition or correction, please contact the page's Responsible Curator directly by e-mail.
| CAZy DB link | |
| http://www.cazy.org/CBM44.html |
Ligand specificities
CBM44, from the CtCel9D-Cel44A enzyme produced by Acetivibrio thermocellus (formerly Clostridium thermocellum), targets ß-1,4-polymers such as xyloglucan and cellulose (hydroxyethylcellulose and Avicel), mixed linkage ß-1,3/ß-1,4- glucans (lichenan and barley) or glucomannan (konjac) [1] and is classified as a type B CBM. Affinity for xylan was very low and binding to laminarin, curdlan, pullulan, pustulan, galactomannan or galactan was negative. Isothermal titration calorimetry (ITC) revealed highest affinity for xyloglucan as a polysaccharide (~81.6 x 104 M-1), which was comparable to the affinity for cellohexaose as an oligosaccharide (~72.8 x 104 M-1). For other cello-oligosaccharides, this affinity decreased with decreasing chain length, while no binding was detected for cellotriose [1].
Structural Features
Like many CBMs, CBM44 exhibits a typical ß-sandwich fold: two antiparallel ß-sheets form a concave and a convex surface. The concave surface forms a deep hydrophobic ligand-binding cleft that is estimated to accommodate up to five glucose residues (~24 Å). Here, three tryptophans (W189, W194 and W198) act as key residues to mediate ligand binding (Figure 1), as confirmed by affinity gel electrophoresis (AGE) and ITC analyses of specific mutants [1]. The orientation of the tryptophans corresponds to the slightly twisted conformation of cello-oligosaccharides in solution [2].
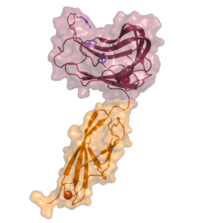
Functionalities
Carbohydrate binding and targeting were described as functions of CBM44. While carbohydrate binding was confirmed by AGE and ITC, the targeting effect was analyzed by the ability of CBM44 to maintain enzyme activity of GH44 (Cel44A) exposed to a substrate mixture [1]. The ensemble of a GH44 and a CBM44 was found in the multimodular cellulase CtCel9D-Cel44A from Acetivibrio thermocellus and ligand spectra of both modules are consistent [1]. In the targeting experiment, the fused GH44-CBM44 construct was compared to the truncated GH44. In separate xyloglucan or carboxymethylcellulose incubations, the activity of GH44-CBM44 was comparable to that of GH44 alone. In a next step, substrate mixtures were used. These mixtures also contained laminarin and pustulan, which were not degraded. While GH44 activity was decreased, activity was restored by GH44-CBM44. Thus, given the complex carbohydrates present in plant cell walls, CBM44 may guide the enzyme to its target sugars. Such effects have also been achieved in synthetic CBM44-enzyme chimeras, e.g. GH12 or GH28 [3, 4].
Family Firsts
- First Identified
- CBM44 was first described as an unknown C-terminal domain in the multimodular cellulase CtCel9D-Cel44A in Acetivibrio thermocellus, formerly designated as CelJ and as Clostridium thermocellum [5, 6]. The protein also features two catalytic domains (GH44 and GH9), a CBM30 and an internal dockerin to target the protein to the cellulosome. The C-terminal region was then identified as having a PKD module as well as a novel CBM binding ß-1,4-glucans, hereby founding the family 44 [1].
- First Structural Characterization
- The same CBM44, together with the preceding PKD module, represents the first structurally characterized candidate of this family (PDB 2c26) [1].
References
- Najmudin S, Guerreiro CI, Carvalho AL, Prates JA, Correia MA, Alves VD, Ferreira LM, Romão MJ, Gilbert HJ, Bolam DN, and Fontes CM. (2006). Xyloglucan is recognized by carbohydrate-binding modules that interact with beta-glucan chains. J Biol Chem. 2006;281(13):8815-28. DOI:10.1074/jbc.M510559200 |
-
Sugiyama H, Hisamichi K, Usui T, Sakai K, Ishiyama J (2000). A study of the conformation of beta-1,4-linked glucose oligomers, cellobiose to cellohexaose, in solution. J Mol Struct. 2000;556(1-3):173-7. DOI: 10.1016/S0022-2860(00)00630-X.
- Furtado GP, Santos CR, Cordeiro RL, Ribeiro LF, de Moraes LA, Damásio AR, Polizeli Mde L, Lourenzoni MR, Murakami MT, and Ward RJ. (2015). Enhanced xyloglucan-specific endo-β-1,4-glucanase efficiency in an engineered CBM44-XegA chimera. Appl Microbiol Biotechnol. 2015;99(12):5095-107. DOI:10.1007/s00253-014-6324-0 |
-
Carli S, Meleiro LP, Salgado JCS, Ward RJ (2022). Synthetic carbohydrate-binding module-endogalacturonase chimeras increase catalytic efficiency and saccharification of lignocellulose residues. Biomass Conv Bioref. 2022. DOI: 10.1007/s13399-022-02716-6.
- Ahsan MM, Kimura T, Karita S, Sakka K, and Ohmiya K. (1996). Cloning, DNA sequencing, and expression of the gene encoding Clostridium thermocellum cellulase CelJ, the largest catalytic component of the cellulosome. J Bacteriol. 1996;178(19):5732-40. DOI:10.1128/jb.178.19.5732-5740.1996 |
- Arai T, Araki R, Tanaka A, Karita S, Kimura T, Sakka K, and Ohmiya K. (2003). Characterization of a cellulase containing a family 30 carbohydrate-binding module (CBM) derived from Clostridium thermocellum CelJ: importance of the CBM to cellulose hydrolysis. J Bacteriol. 2003;185(2):504-12. DOI:10.1128/JB.185.2.504-512.2003 |