CAZypedia needs your help!
We have many unassigned pages in need of Authors and Responsible Curators. See a page that's out-of-date and just needs a touch-up? - You are also welcome to become a CAZypedian. Here's how.
Scientists at all career stages, including students, are welcome to contribute.
Learn more about CAZypedia's misson here and in this article.
Totally new to the CAZy classification? Read this first.
Phosphorylases
- Author: Spencer Williams
- Responsible Curator: Spencer Williams
Overview
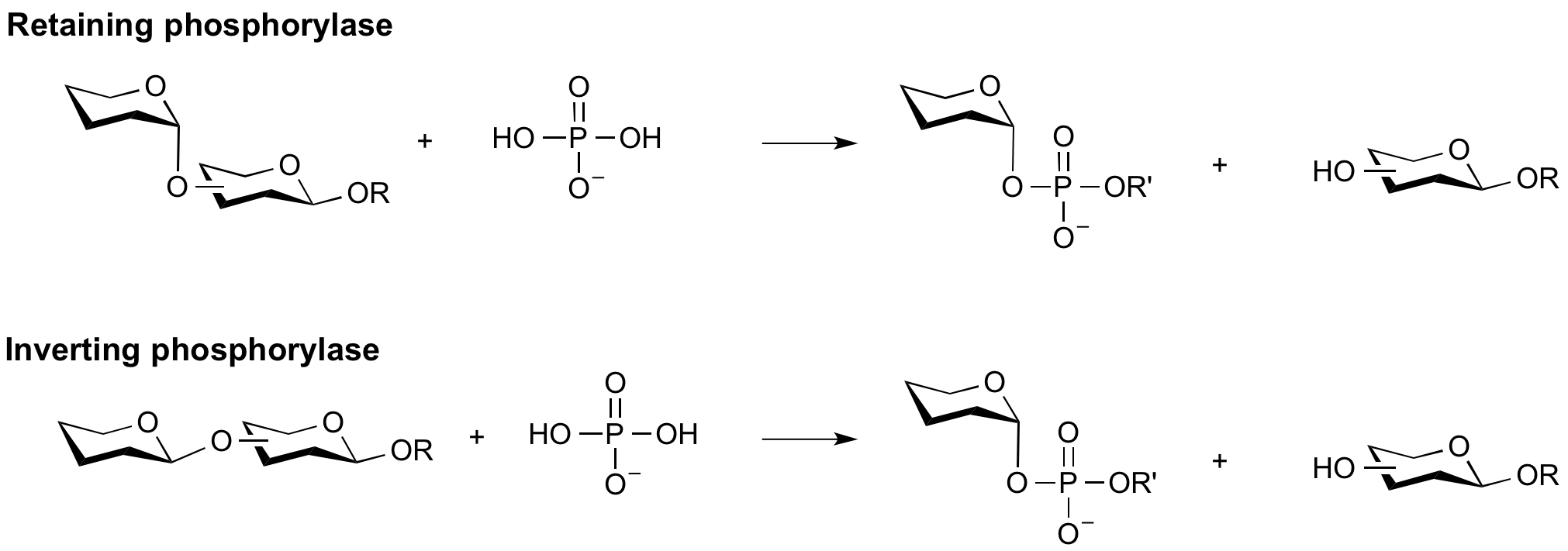
Phosphorylases are enzymes that catalyze the phosphorolysis of the glycosidic bond. They are usually named using a combination of the ‘substrate name’ and ‘phosphorylase’.
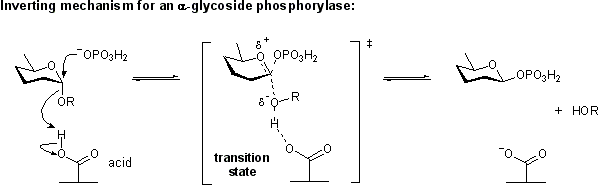
Phosphorolysis of a glycosidic bond can occur with retention or inversion of configuration and always occurs in an exo-fashion leading to formation of a monosaccharide-1-phosphate.
The energy content of the sugar-1-phosphate product means that the cleavage reaction is often in a practical sense reversible and, in Nature, these enzymes may be involved in either synthesis or cleavage of the glycosidic bond. As such there is a relatively fine distinction among sugar phosphorylases, glycoside hydrolases and classical sugar nucleoside (di)phosphate dependent glycosyltransferases. In the last case the synthetic reaction is normally, but not always, irreversible because of the higher energy of a sugar nucleoside (di)phosphate.
Glycogen Phosphorylase
The classical example of a phosphorylase is glycogen phosphorylase. This enzyme catalyzes the cleavage of individual glucosyl residues from glycogen (up to five residues from a branchpoint), forming sequentially deglycosylated glycogen and glucose-1-phosphate. Glycogen phosphorylase has a complex mechanism that is not fully understood and requires pyridoxal phosphate (PLP) as a cofactor. Glycogen phosphorylase is classified into the same sequence-related glycosyltransferase family as starch phosphorylases (Glycosyltransferase family 35), which also require a PLP cofactor.
Other Carbohydrate Phosphorylases
Most sugar phosphorylases act on glucosides and many cleave simple disaccharides such as sucrose, trehalose, cellobiose and maltose leading to glucose-1-phosphate and the other component sugar (glucose or fructose). Other sugar phosphorylases are known that act on chitobiose, laminaribiose, 1,3-beta-glucan and nucleosides.
Mechanism
Sequence and structural analysis of sugar phosphorylases reveal that many have sequences and structures (and likely mechanisms) similar to glycosyltransferases, whereas others have sequences and structures that more closely resemble glycoside hydrolases (Glycoside Hydrolase Family 13, Glycoside Hydrolase Family 65).
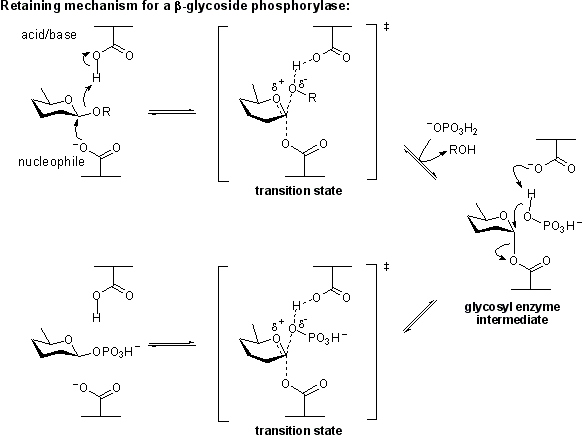
Inverting phosphorylase mechanism
Phosphorolysis of a glycoside with net inversion of anomeric configuration is generally achieved via a one step, single-displacement mechanism involving oxocarbenium ion-like transition states, as shown below. The reaction occurs with acid/base assistance from a suitably positioned carboxylate residue. This mechanism has clear parallels with the mechanism for inverting glycoside hydrolases.
Retaining phosphorylase mechanism
Phosphorolysis of a glycoside with net retention of configuration is achieved via a two step, double-displacement mechanism involving a covalent glycosyl-enzyme intermediate, as shown in the figure below. Each step passes through an oxocarbenium ion-like transition state. Reaction occurs with acid/base and nucleophilic assistance provided by two amino acid side chains, typically glutamate or aspartate. In the first step (called the glycosylation step), one residue plays the role of a nucleophile, attacking the anomeric centre to displace the aglycon and form a glycosyl enzyme intermediate. At the same time the other residue functions as an acid catalyst and protonates the glycosidic oxygen as the bond cleaves. In the second step (known as the deglycosylation step), the glycosyl enzyme is hydrolyzed by phosphate, with the other residue now acting as a base catalyst deprotonating the phosphate as it attacks.