CAZypedia needs your help! We have many unassigned GH, PL, CE, AA, GT, and CBM pages in need of Authors and Responsible Curators.
Scientists at all career stages, including students, are welcome to contribute to CAZypedia. Read more here, and in the 10th anniversary article in Glycobiology.
New to the CAZy classification? Read this first.
*
Consider attending the 15th Carbohydrate Bioengineering Meeting in Ghent, 5-8 May 2024.
Difference between revisions of "Carbohydrate Binding Module Family 4"
Harry Brumer (talk | contribs) m (Text replacement - "\^\^\^(.*)\^\^\^" to "$1") |
|||
| (19 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
<!-- RESPONSIBLE CURATORS: Please replace the {{UnderConstruction}} tag below with {{CuratorApproved}} when the page is ready for wider public consumption --> | <!-- RESPONSIBLE CURATORS: Please replace the {{UnderConstruction}} tag below with {{CuratorApproved}} when the page is ready for wider public consumption --> | ||
| − | {{ | + | {{CuratorApproved}} |
| − | * [[Author]]: | + | * [[Author]]: [[User:Claire Dumon|Claire Dumon]] and [[User:Harry Gilbert|Harry Gilbert]] |
| − | * [[Responsible Curator]]: | + | * [[Responsible Curator]]: [[User:Harry Gilbert|Harry Gilbert]] |
---- | ---- | ||
| Line 17: | Line 17: | ||
== Ligand specificities == | == Ligand specificities == | ||
| − | CBM4 is a bacterial family of protein modules that comprise around 150 amino acids. | + | CBM4 is a bacterial family of protein modules that comprise around 150 amino acids. CBM4 modules target primarily xylans <cite>Abou-Hachem2000</cite>, β1,3-glucans <cite>Boraston2001</cite> or β1,4-glucans <cite>Tomme1996</cite>, although they all display a degree of non-specific binding to other glycans. For example CBM4 members that primarily target xylan or β1,4-glucan also bind to β1,3-β1,4-mixed linked glucans. CBM4s that target predominantly β1,3-glucans also recognise β1,6-glucans <cite>Zverlov2001</cite>. It should also be emphasised that the CBM4 members that preferentially target β1,4-glucans interact with amorphous cellulose but do not bind to crystalline cellulose <cite>Coutinho1992</cite>. |
| − | + | CBM4 modules derived from thermophilic and mesophilic bacteria display affinities (K<sub>D</sub> at room temperature) for their primary ligands of ~1 and ~50 µM, respectively <cite>Boraston2001</cite>. When measured, binding for non-primary ligands was ~100-fold lower than for the target glycan <cite>Abou-Hachem2000</cite>. The affinity for oligosaccharides increased up to the pentaose suggesting the presence of five major sugar binding sites <cite>Abou-Hachem2000,Boraston2001,Johnson1996a</cite>. In all cases isothermal titration calorimetry showed that ligand binding was enthalpically driven and coverage of polysaccharides at saturation indicated an endo-binding mode <cite>Abou-Hachem2000,Boraston2001</cite>. Thus CBM4 is a [[Carbohydrate-binding_modules#Types|type B]] family. | |
== Structural Features == | == Structural Features == | ||
| − | |||
| − | + | [[File:TMCBM4CFCBM4.png|thumb|300px|right|'''Figure 1.''' The U shaped conformation of the binding site of TmCBM4 ([{{PDBlink}}1GUI PDB ID 1GUI]) which binds β1,3-glucan and the linear cleft of CfCBM4 ([{{PDBlink}}1GU3 PDB ID 1GU3]) that recognizes β1,4-glucans. The conserved aromatic resisdues are shown in magenta and the carbon of the ligands in green.]] | |
| − | The crystal structures of TmCBM4-2 and CfCBM4-1, which bind primarily to β1,3-glucan and β1,4-glucan, respectively, were determined in complex with appropriate pentaose ligands <cite>Boraston2002</cite>. The location of the ligand binding cleft, | + | The solution 3D structure of the C-terminal CBM4 module (RmCBM4-2) of the ''Rhodothermus marinus'' xylanase Xyn10A was determined by NMR <cite>Simpson2002</cite>. RmCBM4-2 displays a classical β-jelly-roll fold consisting of two β-sheets comprising five (β-sheet 1) and six (β-sheets) anti-parallel b strands, respectively (Figure 1). RmCBM4-2 is particularly thermostable with a Tm of 97 <sup>o</sup>C which is, in part, caused by two structural calcium ions <cite>Abou-Hachem2002</cite>. The location of the high affinity metal binding site is conserved in all CBMs with a β-sandwich fold <cite>Boraston2004</cite>, while the low affinity calcium site <cite>Abou-Hachem2002</cite> has not been reported in any other family of CBMs. The ligand binding cleft is located on the concave surface (β-sheet 1) and changes in the chemical shifts of NMR spectra upon titrations with xylohexaose indicated that two aromatic residues and a number of polar amino acids interact with ligand in its 3-fold screw-axis conformation <cite>Simpson2002</cite>. |
| + | |||
| + | A crystal structure of a mutant of RmCBM4-2 (X-2; see the Functionalities Section below) in complex with xylopentaose was determined. The data revealed the polar and apolar interactions between the CBM and its ligand, and broadly supports predictions made previously <cite>von-Schantz2012</cite>. Selection of xylan, rather than soluble β1,4-glucans reflects the requirement for a ligand with a precise 3-fold helical conformation, adopted by xylans, as opposed to the twisted orientation of the corresponding glucose polymers. Apart from one C6 hydroxymethyl group, which caused an important arginine to adopt two conformations, the substantial reduction in affinity for gluco-configured ligands was not the result of steric clashes. The structure of an engineered RmCBM4-2 with increased affinity for xyloglucan compared to the wild type protein has been subjected to extensive structural studies <cite>Fisher2015,von-Schantz2012,Gullfot2010</cite>. One of these studies, using neutron crystallography, revealed increased affinity for xyloglucan through the introduction of polar interactions with the xylose side chains of the ligand <cite>Fisher2015</cite>. | ||
| + | |||
| + | The NMR structure of the N-terminal CBM4 module (CfCBM4-1) of the ''Cellulomonas fimi'' endoglucanase CenC <cite>Johnson1996b</cite> revealed a similar β-jelly-roll fold to RmCBM4-2. The location of the ligand binding site comprised the concave surface of β-sheet 1 and aromatic and polar residues involved in ligand recognition were proposed. Crystal structures of TmCBM4-2 and CfCBM4-1, which bind primarily to β1,3-glucan and β1,4-glucan, respectively, were determined in complex with appropriate pentaose ligands <cite>Boraston2002</cite>. The location of the ligand binding cleft, consistent with the NMR structures for RmCBM4-2 and CfCBM4-1, comprises β-sheet 1. A constellation of three aromatic residues, conserved in the two CBMs, form a central aromatic cradle that plays a central role in ligand recognition. These apolar contacts are augmented by a small number of hydrogen bonds with similarly conserved polar amino acids. The importance of these residues was confirmed by spectroscopic and mutagenesis data <cite>Boraston2001,Johnson1996a</cite>. Ligand specificity is conferred by the linear and U-shaped clefts of CfCBM4-1 and TmCBM4-2, respectively, that present optimal topographies for the conformations adopted by β1,4 and β1,3 glucans. | ||
== Functionalities == | == Functionalities == | ||
| − | + | CBM4 members are located in a variety of xylanases and endo-acting glucanases that target β1,3, mixed linked β1,3-β1,4 or β1,4 linkages. In general the specificity of CBM4s are consistent with the substrate specificity of the enzyme, although the four CBM4 modules in a GH16 laminarinase from ''Clostridium thermocellum'' (CtLic16A) bound to a wide range of glycans, many of which share little structural similarities <cite>Dvortsov2012</cite>. An interesting example of this functional relationship between enzyme activity and the specificity of the CBM module is found in a GH16 laminarinase that displays activity against β1,3-glucan (3G) and mixed linked β1,3-β1,4 glucan (34G), consistent with the preference of the N-terminal CBM4 species for 3G and the C-terminal CBM4 module for 34G <cite>Zverlov2001</cite>. The only evidence of increased affinity of CBM4 members through avidity effects is in CtLic16A <cite>Dvortsov2012</cite>. CBM4 modules can also be inserted into the GH10 xylanase catalytic module of Bacteroidetes enzymes <cite>Flint1997</cite>, and this architecture is thought to play a role in enzyme specificity <cite>Dodd2011</cite>. These specific xylanases are mainly encoded by ''Bacteroides'' polysaccharide utilization loci that orchestrate xylan degradation, and these enzymes were suggested to be a functional marker of the utilization of this polysaccharide in the gut <cite>Despres2016</cite>. | |
| − | Using phage display of random mutant libraries a range of specificities have been introduced into the RmCBM4-2 scaffold <cite>Gunnarsson2006a</cite>. In addition to generating variants with increased specificity for xylan <cite>Cicortas-Gunnarsson2007</cite>, mutants of the CBM4 were produced that bound specifically to xyloglucan <cite>von Schantz2009, | + | Using phage display of random mutant libraries a range of specificities have been introduced into the RmCBM4-2 scaffold <cite>Gunnarsson2006a</cite>. In addition to generating variants with increased specificity for xylan <cite>Cicortas-Gunnarsson2007</cite>, mutants of the CBM4 module were produced that bound specifically to xyloglucan <cite>von-Schantz2009,Gunnarsson2006b</cite> and even the protein component of immunoglobulins <cite>Gunnarsson2006c</cite>. The engineered RmCBM4-2 variants have been used to probe plant cell wall architectures <cite>Sandquist2010,Filonova2007</cite>. |
== Family Firsts == | == Family Firsts == | ||
| − | ;First Identified | + | ;First Identified: The N-terminal CBM4 module (CfCBM4-1)from the ''Cellulomonas fimi'' endoglucanase CenC <cite>Coutinho1992</cite>. |
| − | The N-terminal CBM4 (CfCBM4-1)from the ''Cellulomonas fimi'' endoglucanase CenC <cite>Coutinho1992</cite>. | + | ;First Structural Characterization: The first structural characterization of a CBM4 member was a solution NMR structure of CfCBM4-1 <cite>Johnson1996b</cite>. The first crystal structures of a CBM, and the first ligand complex of this family were CfCBM4-1 1 with cellopentaose and TmCBM4-2 with laminariheptaose <cite>Boraston2002</cite>. |
| − | ;First Structural Characterization | + | |
| − | The first structural characterization of a CBM4 was a solution NMR structure of CfCBM4-1 <cite>Johnson1996b</cite>. The first crystal structures of a CBM, and the first ligand complex of this family were CfCBM4-1 and TmCBM4-2 <cite>Boraston2002</cite>. | ||
== References == | == References == | ||
<biblio> | <biblio> | ||
| − | #Abou- | + | #Abou-Hachem2000 pmid=10600638 |
#Boraston2001 pmid=11724582 | #Boraston2001 pmid=11724582 | ||
| Line 58: | Line 60: | ||
#Abou-Hachem2002 pmid=11980476 | #Abou-Hachem2002 pmid=11980476 | ||
| − | #von Schantz2012 pmid=22434778 | + | #von-Schantz2012 pmid=22434778 |
#Fisher2015 pmid=26451738 | #Fisher2015 pmid=26451738 | ||
| − | # Gullfot2010 pmid=19950365 | + | #Gullfot2010 pmid=19950365 |
| + | |||
| + | #Johnson1996b pmid=8916925 | ||
#Boraston2002 pmid=12079353 | #Boraston2002 pmid=12079353 | ||
| Line 68: | Line 72: | ||
#Dvortsov2012 Dvortsov, I. A., Lunina, N. A., Zverlov, V. V., and Velikodvorskaya, G. A. (2012)Properties of four C-terminal carbohydrate-binding modules (CBM4) of laminarinase Lic16A of | #Dvortsov2012 Dvortsov, I. A., Lunina, N. A., Zverlov, V. V., and Velikodvorskaya, G. A. (2012)Properties of four C-terminal carbohydrate-binding modules (CBM4) of laminarinase Lic16A of | ||
Clostridium thermocellum Molecular Biology 46, 817-822 https://doi.org/10.1134/S0026893312060039 | Clostridium thermocellum Molecular Biology 46, 817-822 https://doi.org/10.1134/S0026893312060039 | ||
| + | |||
| + | #Flint1997 pmid=9048892 | ||
| + | |||
| + | #Dodd2011 pmid=21219452 | ||
| + | |||
| + | #Despres2016 pmid=27142817 | ||
#Gunnarsson2006a Gunnarsson, L. C., Karlsson, E. N., Andersson, M., Holst, O., and Ohlin, M. (2006) Molecular engineering of a thermostable carbohydrate-binding module. Biocatalysis and Biotransformation 24, 31-37 https://doi.org/10.1080/10242420500518516 | #Gunnarsson2006a Gunnarsson, L. C., Karlsson, E. N., Andersson, M., Holst, O., and Ohlin, M. (2006) Molecular engineering of a thermostable carbohydrate-binding module. Biocatalysis and Biotransformation 24, 31-37 https://doi.org/10.1080/10242420500518516 | ||
| − | # Cicortas-Gunnarsson2007 pmid=17506724 | + | #Cicortas-Gunnarsson2007 pmid=17506724 |
| − | # von Schantz2009 pmid=19878581 | + | #von-Schantz2009 pmid=19878581 |
#Gunnarsson2006b pmid=16902199 | #Gunnarsson2006b pmid=16902199 | ||
| Line 79: | Line 89: | ||
#Gunnarsson2006c pmid=16427804 | #Gunnarsson2006c pmid=16427804 | ||
| − | #Sandquist2010 Sandquist, D., Filonova, L., von Schantz, L., Ohlin, M., and Daniel, G. (2010)Microdistribution of xyloglucan in differentiating poplar cells. Bioresources 5, 796-807 | + | #Sandquist2010 Sandquist, D., Filonova, L., von Schantz, L., Ohlin, M., and Daniel, G. (2010) Microdistribution of xyloglucan in differentiating poplar cells. Bioresources 5, 796-807 |
| + | |||
| + | #Filonova2007 pmid=17935619 | ||
| − | |||
| − | |||
</biblio> | </biblio> | ||
[[Category:Carbohydrate Binding Module Families|CBM004]] | [[Category:Carbohydrate Binding Module Families|CBM004]] | ||
Latest revision as of 14:18, 18 December 2021
This page has been approved by the Responsible Curator as essentially complete. CAZypedia is a living document, so further improvement of this page is still possible. If you would like to suggest an addition or correction, please contact the page's Responsible Curator directly by e-mail.
| CAZy DB link | |
| http://www.cazy.org/CBM4.html |
Ligand specificities
CBM4 is a bacterial family of protein modules that comprise around 150 amino acids. CBM4 modules target primarily xylans [1], β1,3-glucans [2] or β1,4-glucans [3], although they all display a degree of non-specific binding to other glycans. For example CBM4 members that primarily target xylan or β1,4-glucan also bind to β1,3-β1,4-mixed linked glucans. CBM4s that target predominantly β1,3-glucans also recognise β1,6-glucans [4]. It should also be emphasised that the CBM4 members that preferentially target β1,4-glucans interact with amorphous cellulose but do not bind to crystalline cellulose [5].
CBM4 modules derived from thermophilic and mesophilic bacteria display affinities (KD at room temperature) for their primary ligands of ~1 and ~50 µM, respectively [2]. When measured, binding for non-primary ligands was ~100-fold lower than for the target glycan [1]. The affinity for oligosaccharides increased up to the pentaose suggesting the presence of five major sugar binding sites [1, 2, 6]. In all cases isothermal titration calorimetry showed that ligand binding was enthalpically driven and coverage of polysaccharides at saturation indicated an endo-binding mode [1, 2]. Thus CBM4 is a type B family.
Structural Features
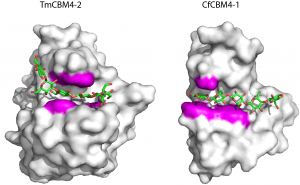
The solution 3D structure of the C-terminal CBM4 module (RmCBM4-2) of the Rhodothermus marinus xylanase Xyn10A was determined by NMR [7]. RmCBM4-2 displays a classical β-jelly-roll fold consisting of two β-sheets comprising five (β-sheet 1) and six (β-sheets) anti-parallel b strands, respectively (Figure 1). RmCBM4-2 is particularly thermostable with a Tm of 97 oC which is, in part, caused by two structural calcium ions [8]. The location of the high affinity metal binding site is conserved in all CBMs with a β-sandwich fold [9], while the low affinity calcium site [8] has not been reported in any other family of CBMs. The ligand binding cleft is located on the concave surface (β-sheet 1) and changes in the chemical shifts of NMR spectra upon titrations with xylohexaose indicated that two aromatic residues and a number of polar amino acids interact with ligand in its 3-fold screw-axis conformation [7].
A crystal structure of a mutant of RmCBM4-2 (X-2; see the Functionalities Section below) in complex with xylopentaose was determined. The data revealed the polar and apolar interactions between the CBM and its ligand, and broadly supports predictions made previously [10]. Selection of xylan, rather than soluble β1,4-glucans reflects the requirement for a ligand with a precise 3-fold helical conformation, adopted by xylans, as opposed to the twisted orientation of the corresponding glucose polymers. Apart from one C6 hydroxymethyl group, which caused an important arginine to adopt two conformations, the substantial reduction in affinity for gluco-configured ligands was not the result of steric clashes. The structure of an engineered RmCBM4-2 with increased affinity for xyloglucan compared to the wild type protein has been subjected to extensive structural studies [10, 11, 12]. One of these studies, using neutron crystallography, revealed increased affinity for xyloglucan through the introduction of polar interactions with the xylose side chains of the ligand [11].
The NMR structure of the N-terminal CBM4 module (CfCBM4-1) of the Cellulomonas fimi endoglucanase CenC [13] revealed a similar β-jelly-roll fold to RmCBM4-2. The location of the ligand binding site comprised the concave surface of β-sheet 1 and aromatic and polar residues involved in ligand recognition were proposed. Crystal structures of TmCBM4-2 and CfCBM4-1, which bind primarily to β1,3-glucan and β1,4-glucan, respectively, were determined in complex with appropriate pentaose ligands [14]. The location of the ligand binding cleft, consistent with the NMR structures for RmCBM4-2 and CfCBM4-1, comprises β-sheet 1. A constellation of three aromatic residues, conserved in the two CBMs, form a central aromatic cradle that plays a central role in ligand recognition. These apolar contacts are augmented by a small number of hydrogen bonds with similarly conserved polar amino acids. The importance of these residues was confirmed by spectroscopic and mutagenesis data [2, 6]. Ligand specificity is conferred by the linear and U-shaped clefts of CfCBM4-1 and TmCBM4-2, respectively, that present optimal topographies for the conformations adopted by β1,4 and β1,3 glucans.
Functionalities
CBM4 members are located in a variety of xylanases and endo-acting glucanases that target β1,3, mixed linked β1,3-β1,4 or β1,4 linkages. In general the specificity of CBM4s are consistent with the substrate specificity of the enzyme, although the four CBM4 modules in a GH16 laminarinase from Clostridium thermocellum (CtLic16A) bound to a wide range of glycans, many of which share little structural similarities [15]. An interesting example of this functional relationship between enzyme activity and the specificity of the CBM module is found in a GH16 laminarinase that displays activity against β1,3-glucan (3G) and mixed linked β1,3-β1,4 glucan (34G), consistent with the preference of the N-terminal CBM4 species for 3G and the C-terminal CBM4 module for 34G [4]. The only evidence of increased affinity of CBM4 members through avidity effects is in CtLic16A [15]. CBM4 modules can also be inserted into the GH10 xylanase catalytic module of Bacteroidetes enzymes [16], and this architecture is thought to play a role in enzyme specificity [17]. These specific xylanases are mainly encoded by Bacteroides polysaccharide utilization loci that orchestrate xylan degradation, and these enzymes were suggested to be a functional marker of the utilization of this polysaccharide in the gut [18].
Using phage display of random mutant libraries a range of specificities have been introduced into the RmCBM4-2 scaffold [19]. In addition to generating variants with increased specificity for xylan [20], mutants of the CBM4 module were produced that bound specifically to xyloglucan [21, 22] and even the protein component of immunoglobulins [23]. The engineered RmCBM4-2 variants have been used to probe plant cell wall architectures [24, 25].
Family Firsts
- First Identified
- The N-terminal CBM4 module (CfCBM4-1)from the Cellulomonas fimi endoglucanase CenC [5].
- First Structural Characterization
- The first structural characterization of a CBM4 member was a solution NMR structure of CfCBM4-1 [13]. The first crystal structures of a CBM, and the first ligand complex of this family were CfCBM4-1 1 with cellopentaose and TmCBM4-2 with laminariheptaose [14].
References
Error fetching PMID 11724582:
Error fetching PMID 8909285:
Error fetching PMID 11238969:
Error fetching PMID 1375311:
Error fetching PMID 8909286:
Error fetching PMID 11980475:
Error fetching PMID 11980476:
Error fetching PMID 22434778:
Error fetching PMID 26451738:
Error fetching PMID 19950365:
Error fetching PMID 8916925:
Error fetching PMID 12079353:
Error fetching PMID 9048892:
Error fetching PMID 21219452:
Error fetching PMID 27142817:
Error fetching PMID 17506724:
Error fetching PMID 19878581:
Error fetching PMID 16902199:
Error fetching PMID 16427804:
Error fetching PMID 17935619:
- Error fetching PMID 10600638:
- Error fetching PMID 11724582:
- Error fetching PMID 8909285:
- Error fetching PMID 11238969:
- Error fetching PMID 1375311:
- Error fetching PMID 8909286:
- Error fetching PMID 11980475:
- Error fetching PMID 11980476:
- Boraston AB, Bolam DN, Gilbert HJ, and Davies GJ. (2004). Carbohydrate-binding modules: fine-tuning polysaccharide recognition. Biochem J. 2004;382(Pt 3):769-81. DOI:10.1042/BJ20040892 |
- Error fetching PMID 22434778:
- Error fetching PMID 26451738:
- Error fetching PMID 19950365:
- Error fetching PMID 8916925:
- Error fetching PMID 12079353:
-
Dvortsov, I. A., Lunina, N. A., Zverlov, V. V., and Velikodvorskaya, G. A. (2012)Properties of four C-terminal carbohydrate-binding modules (CBM4) of laminarinase Lic16A of
Clostridium thermocellum Molecular Biology 46, 817-822 https://doi.org/10.1134/S0026893312060039
- Error fetching PMID 9048892:
- Error fetching PMID 21219452:
- Error fetching PMID 27142817:
-
Gunnarsson, L. C., Karlsson, E. N., Andersson, M., Holst, O., and Ohlin, M. (2006) Molecular engineering of a thermostable carbohydrate-binding module. Biocatalysis and Biotransformation 24, 31-37 https://doi.org/10.1080/10242420500518516
- Error fetching PMID 17506724:
- Error fetching PMID 19878581:
- Error fetching PMID 16902199:
- Error fetching PMID 16427804:
-
Sandquist, D., Filonova, L., von Schantz, L., Ohlin, M., and Daniel, G. (2010) Microdistribution of xyloglucan in differentiating poplar cells. Bioresources 5, 796-807
- Error fetching PMID 17935619: