CAZypedia needs your help! We have many unassigned GH, PL, CE, AA, GT, and CBM pages in need of Authors and Responsible Curators.
Scientists at all career stages, including students, are welcome to contribute to CAZypedia. Read more here, and in the 10th anniversary article in Glycobiology.
New to the CAZy classification? Read this first.
*
Consider attending the 15th Carbohydrate Bioengineering Meeting in Ghent, 5-8 May 2024.
Glycoside Hydrolase Family 82
This page has been approved by the Responsible Curator as essentially complete. CAZypedia is a living document, so further improvement of this page is still possible. If you would like to suggest an addition or correction, please contact the page's Responsible Curator directly by e-mail.
- Authors: ^^^Mirjam Czjzek^^^, ^^^Gurvan Michel^^^, and ^^^Etienne Rebuffet^^^
- Responsible Curator: ^^^Mirjam Czjzek^^^
| Glycoside Hydrolase Family GH82 | |
| Clan | none |
| Mechanism | inverting |
| Active site residues | known |
| CAZy DB link | |
| http://www.cazy.org/fam/GH82.html | |
Substrate specificities
The two known members of glycoside hydrolase family 82 enzymes cleave the β-1,4 galactosidic bond of the marine algal polysaccharide iota-carrageenan [1] yielding products of the neocarrabiose series.
Kinetics and Mechanism
Family 82 enzymes are inverting enzymes, as first shown by NMR [1] on the iota-carrageenase from Alteromonas fortis.
Catalytic Residues
From structural analysis the catalytic residues have been predicted to be two out of the three candidate amino acids Glu245, Asp247 or Glu310 in the A. fortis iota-carrageenase [2]. A study in 2010, which utilized site directed mutagenesis, has confirmed that Glu245 plays the role of the general acid residue in this inverting enzyme, while Asp247 is the general base activating the nucleophilic water molecule [3]. However, intriguingly the position of equivalent residues to Asp247 in other iota-carrageenase sequences are not strictly conserved [3, 4].
Three-dimensional structures
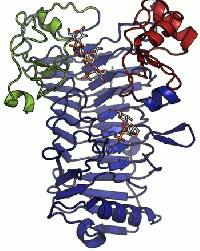
A crystal structure has only been determined for the iota-carrageenase from A. fortis [5]. The crystal structure of a product complex has shed light on the existance of domain movement of domain A that is closed around the oligo-carrageenan in the complexed form and open in the uncomplexed enzyme (Figure 1) [6].
Family Firsts
- First sequence identification and family creation
- iota-carrageenase sequences have been first reported for enzymes from A. fortis and Z. galactanivorans [1].
- First sterochemistry determination
- GH82 enzymes are inverting as shown by NMR [1].
- First general acid residue identification
- Glu245 [3]
- First general base residue identification
- Asp247 [3].
- First 3-D structure
- iota-carrageenase from A. fortis [2]. The structure belongs to the β-helix fold (PDB 1h80 and PDB 1ktw).
References
- Barbeyron T, Michel G, Potin P, Henrissat B, and Kloareg B. (2000). iota-Carrageenases constitute a novel family of glycoside hydrolases, unrelated to that of kappa-carrageenases. J Biol Chem. 2000;275(45):35499-505. DOI:10.1074/jbc.M003404200 |
- Michel G, Chantalat L, Fanchon E, Henrissat B, Kloareg B, and Dideberg O. (2001). The iota-carrageenase of Alteromonas fortis. A beta-helix fold-containing enzyme for the degradation of a highly polyanionic polysaccharide. J Biol Chem. 2001;276(43):40202-9. DOI:10.1074/jbc.M100670200 |
- Rebuffet E, Barbeyron T, Jeudy A, Jam M, Czjzek M, and Michel G. (2010). Identification of catalytic residues and mechanistic analysis of family GH82 iota-carrageenases. Biochemistry. 2010;49(35):7590-9. DOI:10.1021/bi1003475 |
- Hatada Y, Mizuno M, Li Z, and Ohta Y. (2011). Hyper-production and characterization of the ι-carrageenase useful for ι-carrageenan oligosaccharide production from a deep-sea bacterium, Microbulbifer thermotolerans JAMB-A94T, and insight into the unusual catalytic mechanism. Mar Biotechnol (NY). 2011;13(3):411-22. DOI:10.1007/s10126-010-9312-0 |
- Michel G, Helbert W, Kahn R, Dideberg O, and Kloareg B. (2003). The structural bases of the processive degradation of iota-carrageenan, a main cell wall polysaccharide of red algae. J Mol Biol. 2003;334(3):421-33. DOI:10.1016/j.jmb.2003.09.056 |