CAZypedia needs your help!
We have many unassigned pages in need of Authors and Responsible Curators. See a page that's out-of-date needs a touch-up? - you are welcome to become a CAZypedian. Here's how.
Scientists at all career stages, including students, are welcome to contribute
Read more about CAZypedia here, and in the 10th anniversary article in Glycobiology.
New to the CAZy classification? Read this first.
Difference between revisions of "Transition state"
| Line 3: | Line 3: | ||
---- | ---- | ||
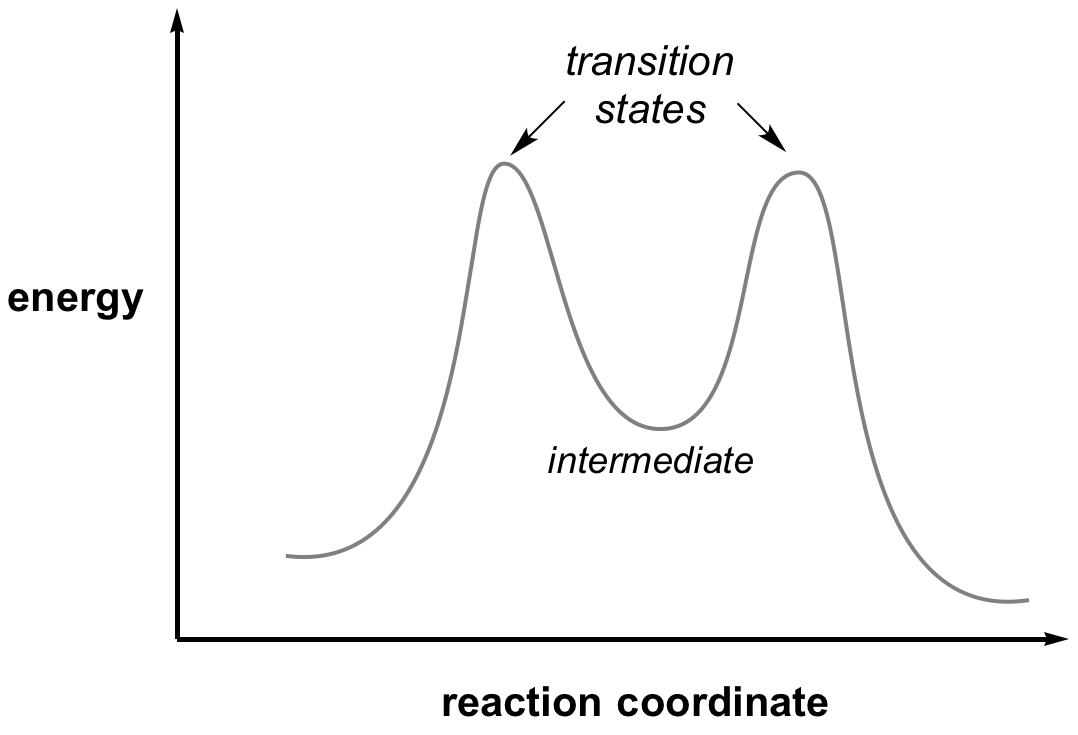
| − | The transition state of any reaction is the highest point in the reaction coordinate, this is a balance point between starting material and product. By definition it does not have a lifetime, and so it cannot be isolated and studied directly. However, transition state structures can be determined by the measurement of kinetic isotope effects. | + | The '''transition state''' of any reaction is the highest point in the reaction coordinate, this is a balance point between starting material and product. By definition it does not have a lifetime, and so it cannot be isolated and studied directly. However, transition state structures can be determined by the measurement of kinetic isotope effects. |
[[Image:ReactionCoordinate.png|centre]] | [[Image:ReactionCoordinate.png|centre]] | ||
[[Category:Definitions and explanations]] | [[Category:Definitions and explanations]] | ||
Revision as of 05:17, 10 July 2009
- Author: Stephen Withers
- Responsible Editor: Spencer Williams
The transition state of any reaction is the highest point in the reaction coordinate, this is a balance point between starting material and product. By definition it does not have a lifetime, and so it cannot be isolated and studied directly. However, transition state structures can be determined by the measurement of kinetic isotope effects.