CAZypedia needs your help! We have many unassigned GH, PL, CE, AA, GT, and CBM pages in need of Authors and Responsible Curators.
Scientists at all career stages, including students, are welcome to contribute to CAZypedia. Read more here, and in the 10th anniversary article in Glycobiology.
New to the CAZy classification? Read this first.
*
Consider attending the 15th Carbohydrate Bioengineering Meeting in Ghent, 5-8 May 2024.
Difference between revisions of "Glycoside Hydrolase Family 73"
| Line 31: | Line 31: | ||
The GH73 family is comprised of bacterial or prokaryotic viral members. Most of the enzymes of this family are peptidoglycan hydrolases that cleave the β-1,4-glycosidic linkage between N-acetylglucosaminyl (NAG) and N-acetylmuramyl (NAM) moieties in the carbohydrate backbone of bacterial peptidoglycan. | The GH73 family is comprised of bacterial or prokaryotic viral members. Most of the enzymes of this family are peptidoglycan hydrolases that cleave the β-1,4-glycosidic linkage between N-acetylglucosaminyl (NAG) and N-acetylmuramyl (NAM) moieties in the carbohydrate backbone of bacterial peptidoglycan. | ||
| − | The activity of the GH73 is mainly focused in daughter cell separation during vegetative growth and it is very often involved in the hydrolysis of the septum after cell division (Acp from | + | The activity of the GH73 is mainly focused in daughter cell separation during vegetative growth and it is very often involved in the hydrolysis of the septum after cell division (Acp from ''Clotridium Perfringens'' (20190047( 1)) AltA from ''Enterococcus faecalis''(17041059 (2)). Only ''Listeria monocytogene'' uses Auto as a virulence-associated peptidoglycan hydrolase for host-cell invasion (ref (3)). The GH73 are mostly surface located and exhibit repeated sequences that could be involved in cell-wall binding and therefore reinforce the enzymes catalytic activity. Unknown repeated domains are appended for instance to LytD and LytG from Bacillus subtilis (ref/ref), AcmB from Lactococcus lactis (ref) and Auto from L. monocytogene. Some of these repeated domains have been identified like CBM50 also known as LysM domain appended to AcmA for Lactococcus lactis (ref), AltA from Enterococcus faecalis (ref) and Mur2-Mur2 from Enterococcus hirae(ref). |
== Kinetics and Mechanism == | == Kinetics and Mechanism == | ||
Revision as of 08:43, 27 July 2010
This page is currently under construction. This means that the Responsible Curator has deemed that the page's content is not quite up to CAZypedia's standards for full public consumption. All information should be considered to be under revision and may be subject to major changes.
- Author: ^^^Florence Vincent^^^
- Responsible Curator: ^^^Bernard Henrissat^^^
| Glycoside Hydrolase Family GH73 | |
| Clan | none, α+β "lysozyme fold" |
| Mechanism | not known |
| Active site residues | partially known |
| CAZy DB link | |
| http://www.cazy.org/GH73.html | |
Substrate specificities
The GH73 family is comprised of bacterial or prokaryotic viral members. Most of the enzymes of this family are peptidoglycan hydrolases that cleave the β-1,4-glycosidic linkage between N-acetylglucosaminyl (NAG) and N-acetylmuramyl (NAM) moieties in the carbohydrate backbone of bacterial peptidoglycan.
The activity of the GH73 is mainly focused in daughter cell separation during vegetative growth and it is very often involved in the hydrolysis of the septum after cell division (Acp from Clotridium Perfringens (20190047( 1)) AltA from Enterococcus faecalis(17041059 (2)). Only Listeria monocytogene uses Auto as a virulence-associated peptidoglycan hydrolase for host-cell invasion (ref (3)). The GH73 are mostly surface located and exhibit repeated sequences that could be involved in cell-wall binding and therefore reinforce the enzymes catalytic activity. Unknown repeated domains are appended for instance to LytD and LytG from Bacillus subtilis (ref/ref), AcmB from Lactococcus lactis (ref) and Auto from L. monocytogene. Some of these repeated domains have been identified like CBM50 also known as LysM domain appended to AcmA for Lactococcus lactis (ref), AltA from Enterococcus faecalis (ref) and Mur2-Mur2 from Enterococcus hirae(ref).
Kinetics and Mechanism
Content is to be added here.
Catalytic Residues
Content is to be added here.
Three-dimensional structures
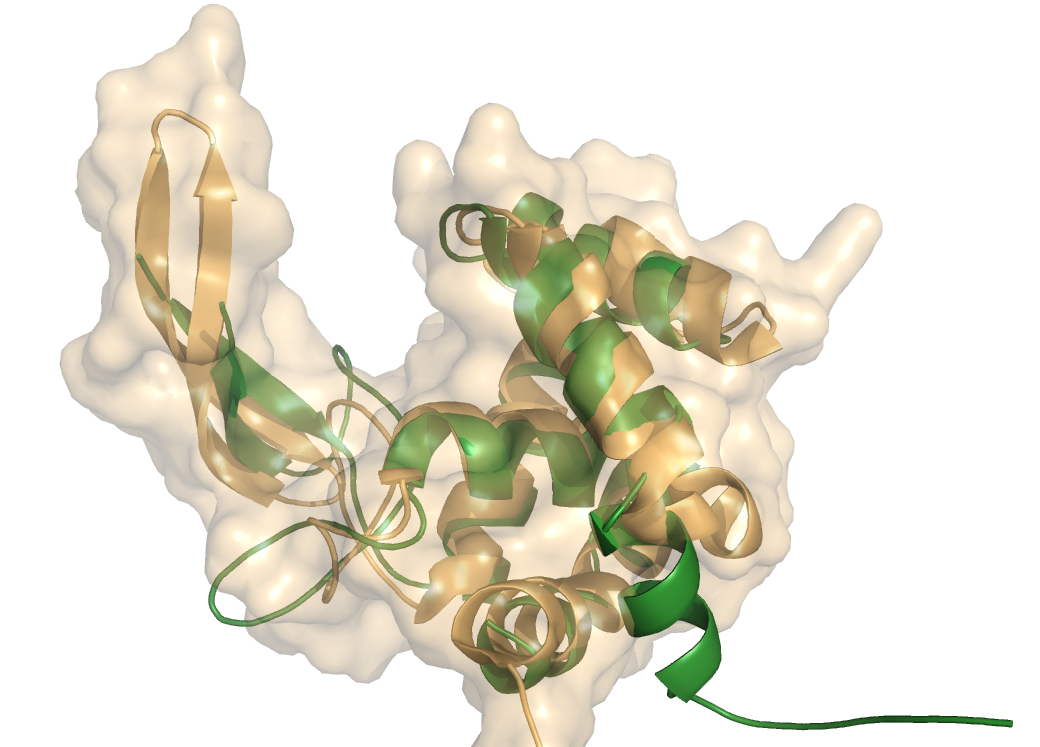
Crystal structure of GH73 are available and have been coincidently reported, FlgJ from Sphingomonas sp. (SPH1045-C) [1] and Auto a virulence associated peptigoglycan hydrolase from Listeria monocytogenes [2]. A structure for a catalytic mutant (E185A) of FlgJ has been solved by Maruyama et al [3] but doesn’t show any conformational changes. The two GH73 show the same fold, with two subdomains consisting of a β-lobe and an α-lobe that together create an extended substrate binding groove (Figure 1). With a typical lysozyme (α+β) fold, the catalytic domain of Auto is structurally related to the catalytic domain of Slt70 from E. coli [4], the family GH19 chitinases and goose egg-white lysozyme (GEWL, GH23)[5]. FlgJ is structurally related to a peptidoglycan degrading enzyme from the bacteriophage phi 29 [6] and also to family GH22 and GH23 lysozymes.
Family Firsts
- First stereochemistry determination
- Cite some reference here, with a short (1-2 sentence) explanation
- First catalytic nucleophile identification
- Cite some reference here, with a short (1-2 sentence) explanation
- First general acid/base residue identification
- Cite some reference here, with a short (1-2 sentence) explanation
- First 3-D structure
- Cite some reference here, with a short (1-2 sentence) explanation
References
- Hashimoto W, Ochiai A, Momma K, Itoh T, Mikami B, Maruyama Y, and Murata K. (2009). Crystal structure of the glycosidase family 73 peptidoglycan hydrolase FlgJ. Biochem Biophys Res Commun. 2009;381(1):16-21. DOI:10.1016/j.bbrc.2009.01.186 |
- Bublitz M, Polle L, Holland C, Heinz DW, Nimtz M, and Schubert WD. (2009). Structural basis for autoinhibition and activation of Auto, a virulence-associated peptidoglycan hydrolase of Listeria monocytogenes. Mol Microbiol. 2009;71(6):1509-22. DOI:10.1111/j.1365-2958.2009.06619.x |
- Maruyama Y, Ochiai A, Itoh T, Mikami B, Hashimoto W, and Murata K. (2010). Mutational studies of the peptidoglycan hydrolase FlgJ of Sphingomonas sp. strain A1. J Basic Microbiol. 2010;50(4):311-7. DOI:10.1002/jobm.200900249 |
- van Asselt EJ, Thunnissen AM, and Dijkstra BW. (1999). High resolution crystal structures of the Escherichia coli lytic transglycosylase Slt70 and its complex with a peptidoglycan fragment. J Mol Biol. 1999;291(4):877-98. DOI:10.1006/jmbi.1999.3013 |
- Weaver LH, Grütter MG, and Matthews BW. (1995). The refined structures of goose lysozyme and its complex with a bound trisaccharide show that the "goose-type" lysozymes lack a catalytic aspartate residue. J Mol Biol. 1995;245(1):54-68. DOI:10.1016/s0022-2836(95)80038-7 |
- Xiang Y, Morais MC, Cohen DN, Bowman VD, Anderson DL, and Rossmann MG. (2008). Crystal and cryoEM structural studies of a cell wall degrading enzyme in the bacteriophage phi29 tail. Proc Natl Acad Sci U S A. 2008;105(28):9552-7. DOI:10.1073/pnas.0803787105 |