CAZypedia needs your help! We have many unassigned GH, PL, CE, AA, GT, and CBM pages in need of Authors and Responsible Curators.
Scientists at all career stages, including students, are welcome to contribute to CAZypedia. Read more here, and in the 10th anniversary article in Glycobiology.
New to the CAZy classification? Read this first.
*
Consider attending the 15th Carbohydrate Bioengineering Meeting in Ghent, 5-8 May 2024.
Difference between revisions of "Glycosyltransferases"
| Line 26: | Line 26: | ||
=== Sequence based classification === | === Sequence based classification === | ||
| − | Sequence-based classification uses algorithmic methods to assign sequences to various families. The glycosyltransferases have been classified into more than 90 families <cite>Campbell1997 Coutinho2003</cite>; this is permanently available through the Carbohydrate Active enZyme database | + | Sequence-based classification uses algorithmic methods to assign sequences to various families. The glycosyltransferases have been classified into more than 90 families <cite>Campbell1997 Coutinho2003</cite>; this is permanently available through the Carbohydrate Active enZyme database <cite>CAZyURL</cite>. Each family (GT family) contains proteins that are related by sequence, and by corollary, fold. This allows several useful predictions to be made since the catalytic machinery is conserved within each family. Usually, the mechanism used (ie retaining or inverting) is conserved within a GT family. |
| − | |||
| − | |||
=== 3-D folds === | === 3-D folds === | ||
| − | Sugar nucleotide-dependent glycosyltransferases | + | Sugar nucleotide-dependent glycosyltransferases have been found to possess only two different folds, termed the GT-A and GT-B folds. The GT-A fold is typified by the first member to have its X-ray structure determined, SpsA from ''Bacillus subtilus'' <cite>Charnock1999</cite>. |
{|name="userboxes" id="userboxes" style="margin-left: 1em; margin-bottom: 0.5em; width: 170px; border: {{{bordercolor|#99B3FF}}} solid 1px; background-color: {{{backgroundcolor|#FFFFFF}}}; {{{extra-css|}}}" align="right" | {|name="userboxes" id="userboxes" style="margin-left: 1em; margin-bottom: 0.5em; width: 170px; border: {{{bordercolor|#99B3FF}}} solid 1px; background-color: {{{backgroundcolor|#FFFFFF}}}; {{{extra-css|}}}" align="right" | ||
|- | |- | ||
| Line 46: | Line 44: | ||
</center> | </center> | ||
|} | |} | ||
| + | The GT-B fold is exemplified by its first member, the beta-glucosyltransferase from bacteriophage T4 <cite>Vrielink1994</cite>. | ||
| + | |||
{|name="userboxes" id="userboxes" style="margin-left: 1em; margin-bottom: 0.5em; width: 170px; border:{{{bordercolor|#99B3FF}}} solid 1px; background-color:{{{backgroundcolor|#FFFFFF}}}; {{{extra-css|}}}" align="right" | {|name="userboxes" id="userboxes" style="margin-left: 1em; margin-bottom: 0.5em; width: 170px; border:{{{bordercolor|#99B3FF}}} solid 1px; background-color:{{{backgroundcolor|#FFFFFF}}}; {{{extra-css|}}}" align="right" | ||
|- | |- | ||
Revision as of 03:49, 15 August 2010
This page is currently under construction. This means that the Responsible Curator has deemed that the page's content is not quite up to CAZypedia's standards for full public consumption. All information should be considered to be under revision and may be subject to major changes.
- Author: Spencer Williams
- Responsible Curator: Spencer Williams
Overview
Glycosyltransferases are enzymes that catalyze the formation of the glycosidic linkage to form a glycoside. These enzymes utilize 'activated' sugar phosphates as glycosyl donors, and catalyze glycosyl group transfer to a nucleophilic group, usually an alcohol. The product of glycosyl transfer may be an O-, N-, S-, or C-glycoside; the glycoside may be part of a monosaccharide glycoside, oligosaccharide, or polysaccharide ([1, 2, 3, 4, 5]).
Donors
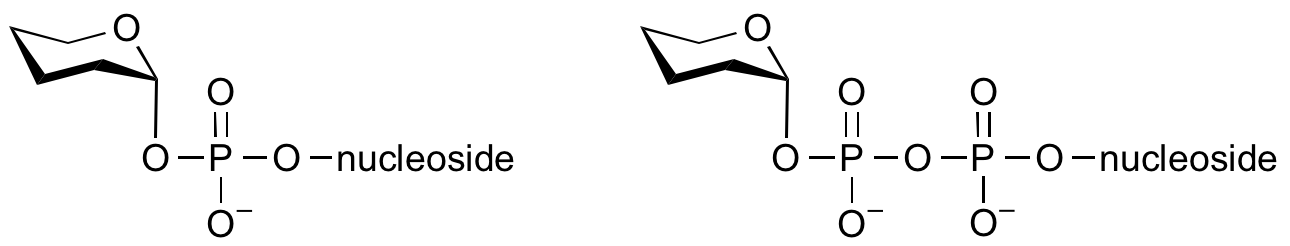
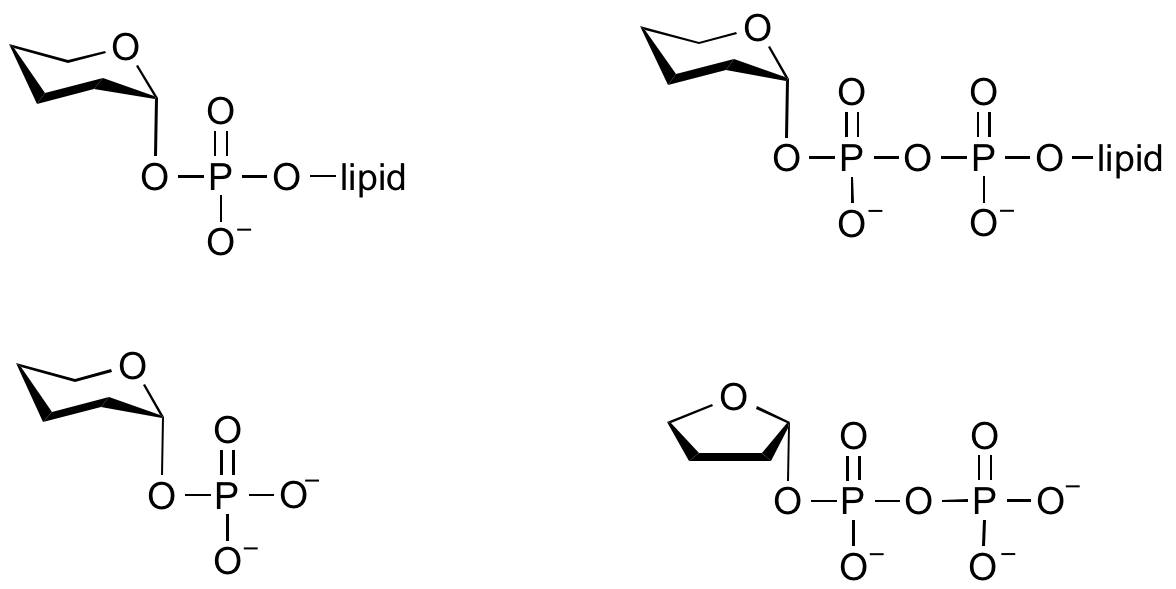
Glycosyltransferases can utilize a range of donor species. Sugar mono- or diphosphonucleotides are sometimes termed Leloir donors (after Nobel prize winner, Luis Leloir); the corresponding enzymes are termed Leloir donors.
Glycosyltransferases that utilize non-nucleotide donors, which may be polyprenol pyrophosphates, polyprenol phosphates, sugar-1-phosphates, or sugar-1-pyrophosphates, are termed non-Leloir glycosyltransferases.
Mechanism
Glycosyltransferases catalyze the transfer of glycosyl groups to a nucleophilic acceptor with either retention or inversion of configuration at the anomeric centre. This allows the classification of glycosyltransferases as either retaining or inverting enzymes.
Inverting glycosyltransferases
Structural and kinetic data for inverting glycosyltransferases support a mechanism that proceeds through a single nucleophilic substitution step, facilitated by an enzymic general base catalyst. The transition state is believed to possess substantial oxocarbenium ion character.
Retaining glycosyltransferases
Classification
Sequence based classification
Sequence-based classification uses algorithmic methods to assign sequences to various families. The glycosyltransferases have been classified into more than 90 families [6, 7]; this is permanently available through the Carbohydrate Active enZyme database [8]. Each family (GT family) contains proteins that are related by sequence, and by corollary, fold. This allows several useful predictions to be made since the catalytic machinery is conserved within each family. Usually, the mechanism used (ie retaining or inverting) is conserved within a GT family.
3-D folds
Sugar nucleotide-dependent glycosyltransferases have been found to possess only two different folds, termed the GT-A and GT-B folds. The GT-A fold is typified by the first member to have its X-ray structure determined, SpsA from Bacillus subtilus [9].
<jmol> <jmolApplet> <color>white</color> <frame>true</frame> <uploadedFileContents>1h7l.pdb</uploadedFileContents> <script>cpk off; wireframe off; cartoon; color cartoon powderblue; select ligand; wireframe 0.3; select MG; spacefill;set spin Y 10; spin on; set antialiasDisplay ON</script> </jmolApplet> </jmol> |
The GT-B fold is exemplified by its first member, the beta-glucosyltransferase from bacteriophage T4 [10].
<jmol> <jmolApplet> <color>white</color> <frame>true</frame> <uploadedFileContents>1bgt.pdb</uploadedFileContents> <script>cpk off; wireframe off; cartoon; color cartoon powderblue; select ligand; wireframe 0.3; select MG; spacefill;set spin Y 10; spin on; set antialiasDisplay ON</script> </jmolApplet> </jmol> |
Role of metals
Common sugar nucleotide donors
References
- Robert V. Stick and Spencer J. Williams. (2009) Carbohydrates. Elsevier Science.
- Lairson LL, Henrissat B, Davies GJ, and Withers SG. (2008). Glycosyltransferases: structures, functions, and mechanisms. Annu Rev Biochem. 2008;77:521-55. DOI:10.1146/annurev.biochem.76.061005.092322 |
- Coutinho PM, Deleury E, Davies GJ, and Henrissat B. (2003). An evolving hierarchical family classification for glycosyltransferases. J Mol Biol. 2003;328(2):307-17. DOI:10.1016/s0022-2836(03)00307-3 |
Chapter 5: Coutinho PM, Rancurel C, Stam M, Bernard T, Couto FM, Danchin EGJ, Henrissat B. "Carbohydrate-active Enzymes Database: Principles and Classification of Glycosyltransferases."
- Campbell JA, Davies GJ, Bulone V, and Henrissat B. (1997). A classification of nucleotide-diphospho-sugar glycosyltransferases based on amino acid sequence similarities. Biochem J. 1997;326 ( Pt 3)(Pt 3):929-39. DOI:10.1042/bj3260929u |
- Claus-Wilhelm von der Lieth, Thomas Luetteke, and Martin Frank. (2010-01-19) Bioinformatics for Glycobiology and Glycomics: An Introduction. Wiley.
- Campbell JA, Davies GJ, Bulone V, and Henrissat B. (1997). A classification of nucleotide-diphospho-sugar glycosyltransferases based on amino acid sequence similarities. Biochem J. 1997;326 ( Pt 3)(Pt 3):929-39. DOI:10.1042/bj3260929u |
- Coutinho PM, Deleury E, Davies GJ, and Henrissat B. (2003). An evolving hierarchical family classification for glycosyltransferases. J Mol Biol. 2003;328(2):307-17. DOI:10.1016/s0022-2836(03)00307-3 |
- Charnock SJ and Davies GJ. (1999). Structure of the nucleotide-diphospho-sugar transferase, SpsA from Bacillus subtilis, in native and nucleotide-complexed forms. Biochemistry. 1999;38(20):6380-5. DOI:10.1021/bi990270y |
- Vrielink A, Rüger W, Driessen HP, and Freemont PS. (1994). Crystal structure of the DNA modifying enzyme beta-glucosyltransferase in the presence and absence of the substrate uridine diphosphoglucose. EMBO J. 1994;13(15):3413-22. DOI:10.1002/j.1460-2075.1994.tb06646.x |