CAZypedia needs your help!
We have many unassigned pages in need of Authors and Responsible Curators. See a page that's out-of-date and just needs a touch-up? - You are also welcome to become a CAZypedian. Here's how.
Scientists at all career stages, including students, are welcome to contribute.
Learn more about CAZypedia's misson here and in this article.
Totally new to the CAZy classification? Read this first.
Difference between revisions of "Reaction intermediate"
(New page: * Author: Stephen Withers * Responsible Editor: Spencer Williams ---- An '''intermediate''' in a reaction is a species formed that has a real...) |
Harry Brumer (talk | contribs) |
||
| Line 1: | Line 1: | ||
| − | |||
| − | |||
* Author: [[User:Withers|Stephen Withers]] | * Author: [[User:Withers|Stephen Withers]] | ||
| − | * Responsible | + | * Responsible Curator: [[User:SpencerWilliams|Spencer Williams]] |
---- | ---- | ||
Revision as of 16:33, 27 July 2009
- Author: Stephen Withers
- Responsible Curator: Spencer Williams
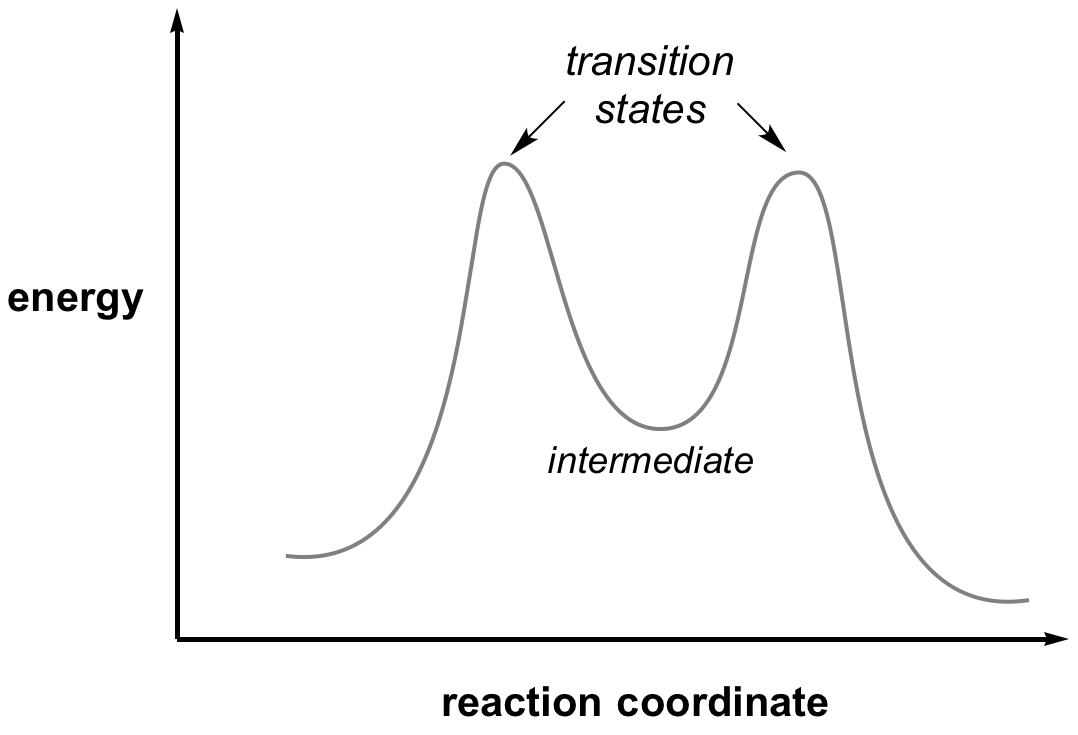
An intermediate in a reaction is a species formed that has a real lifetime, best defined as being greater than the time for a bond vibration (approx 10-12 sec). This will correspond to a minimum in the reaction coordinate diagram located between the reactant and product. Depending on their lifetimes and the tools available, intermediates can sometimes be trapped and observed by physical and chemical techniques.