CAZypedia needs your help! We have many unassigned GH, PL, CE, AA, GT, and CBM pages in need of Authors and Responsible Curators.
Scientists at all career stages, including students, are welcome to contribute to CAZypedia. Read more here, and in the 10th anniversary article in Glycobiology.
New to the CAZy classification? Read this first.
*
Consider attending the 15th Carbohydrate Bioengineering Meeting in Ghent, 5-8 May 2024.
Difference between revisions of "Glycoside Hydrolase Family 18"
Harry Brumer (talk | contribs) m (Text replacement - "\^\^\^(.*)\^\^\^" to "$1") |
|||
| (52 intermediate revisions by 5 users not shown) | |||
| Line 1: | Line 1: | ||
<!-- CURATORS: Please replace the {{UnderConstruction}} tag below with {{CuratorApproved}} when the page is ready for wider public consumption --> | <!-- CURATORS: Please replace the {{UnderConstruction}} tag below with {{CuratorApproved}} when the page is ready for wider public consumption --> | ||
{{CuratorApproved}} | {{CuratorApproved}} | ||
| − | * [[Author]]s: | + | * [[Author]]s: [[User:Gideon Davies|Gideon Davies]], [[User:Nathalie Juge|Nathalie Juge]], [[User:Vincent Eijsink|Vincent Eijsink]] |
| − | * [[Responsible Curator]]: | + | * [[Responsible Curator]]: [[User:Gideon Davies|Gideon Davies]] |
---- | ---- | ||
| Line 22: | Line 22: | ||
|{{Hl2}} colspan="2" align="center" |'''CAZy DB link''' | |{{Hl2}} colspan="2" align="center" |'''CAZy DB link''' | ||
|- | |- | ||
| − | | colspan="2" | | + | | colspan="2" |{{CAZyDBlink}}GH18.html |
|} | |} | ||
</div> | </div> | ||
| Line 29: | Line 29: | ||
== Substrate specificities == | == Substrate specificities == | ||
| − | GH18 is unusual in having both catalytically active | + | Family GH18 is unusual in having [[glycoside hydrolases]] that are both catalytically active chitinases (EC 3.2.1.14) and endo-β-N-acetylglucosaminidases (EC 3.2.1.96) and also sub-families of non-hydrolytic proteins that function as carbohydrate binding modules / "lectins" or as xylanase inhibitors. |
| + | The active chitinases comprise non-processive endo-acting enzymes as well as processive enzymes with exo- and endo-binding preferences <cite>Horn2006a,Hult2005</cite>. Most enzymes primarily produce chitobiose, but some endo-acting family 18 chitinases are not capable of cleaving trimers or tetramers and thus yield longer products. Note that in older literature the endo-/exo-character of these enzymes often is assessed by studying the degradation of oligomeric substrates, and that several recent studies have shown this method to be invalid. | ||
| + | Family 18 chitinases break down all forms of chitin at varying rates depending on the enzyme and the substrate. They also act on chitosan with degrees of acetylation as low as 13 % <cite>Sorbotten2005</cite> and some are known to degrade peptidoglycan <cite>Bokma1997</cite>. Studies on family 18 chitinases from ''Serratia marcescens'' have suggested that most subsites for sugar binding show some promiscuity with the notable exception of subsite -1 <cite>Horn2006a</cite>. | ||
== Kinetics and Mechanism == | == Kinetics and Mechanism == | ||
| − | GH18 enzymes belong to a | + | GH18 enzymes perform enzymic catalysis with [[retaining|retention]] of anomeric configuration. They belong to a group of enzymes (including GH families 18, [[Glycoside Hydrolase Family 20|20]], [[Glycoside Hydrolase Family 25|25]], [[Glycoside Hydrolase Family 56|56]], [[Glycoside Hydrolase Family 84|84]], and [[Glycoside Hydrolase Family 85|85]]) that perform catalysis using a double-displacement reaction but instead of the more common enzyme-derived nucleophile they utilize the ''N''-acetamido carbonyl oxygen in what is termed [[neighboring group participation]] (or substrate participation or anchimeric assistance). Figures showing such a mechanism date back to Koshland's 1953 review <cite>Koshland1953</cite>; indeed they frequent the chemical literature of participating groups long before that, but it is primarily through the work on GH18 <cite>TerwisschavanScheltinga1995</cite> and soon after [[GH20]] <cite>Tews1996,Armand1997</cite> that such a mechanism became well established. In such a mechanism, which occurs with (net) retention of anomeric configuration, the enzyme provides a [[general acid]] function to protonate the leaving group to facilitate its departure with the substrate carbonyl oxygen playing the role of nucleophile to generate a bicyclic "[[oxazolinium ion]]" [[intermediate]], which subsequently breaks down following the microscopic reverse ''via'' hydrolysis or occasionally transglycosylation). Such a mechanism has a number of facets, one of which is its potential inhibition using thiazolines <cite>Macdonald</cite>. Allosamidin, a pseudotrisaccharide consisting of two ''N''-acetylallosamine sugars linked to an allosamizoline moiety <cite>Sakuda1987</cite>, is a well known natural compound that is a high affinity inhibitor of family 18 chitinases (see Figure). |
| + | [[Image:Ligands.PNG|thumb|right|300px|'''Trisaccharide oxazolinium ion intermediate (upper panel), allosamidin (middle panel), and trisaccharide thiazoline (lower panel).''']] Like in many other enzymes acting on polysaccharides the substrate-binding clefts of processive family 18 chitinases are lined with aromatic residues. Family 18 chitinases have proven very useful to gain insight into the structural basis of a processive mechanism and in the importance of such a mechanism for biomass-converting efficiency <cite>Eijsink2008,Zakariassen2009</cite>. As previously suggested for cellulases in e.g. <cite>Varrot2003</cite>, aromatic residues close to the catalytic center play a crucial role in processivity <cite>Horn2006b</cite>. | ||
== Catalytic Residues == | == Catalytic Residues == | ||
| − | The catalytically active GH18 enzymes use a double displacement reaction mechanism with | + | The catalytically-active GH18 enzymes use a double displacement reaction mechanism with [[neighboring group participation]](see Figure). In this mechanism the carbonyl oxygen of the substrate acts as a nucleophile, with assistance from a carboxylate (Asp) that acts to deprotonate the ''N''-acetamido nitrogen during [[oxazolinium ion]] formation/breakdown. A second catalytic acid residue (glutamate in family GH18, but often also Asp in other families using this mechanism) acts as a general acid/base to protonate the glycosidic oxygen to assist in the departure of the aglycon, and to deprotonate the nucleophilic water molecule during the hydrolysis of the [[oxazolinium ion]] [[intermediate]]. In family GH18 the two catalytic carboxylates are found in an D-X-E motif whereas in other families the carboxylates may be adjacent, such as the DD motif in family [[GH84]] (for example see <cite>He2010</cite>). The physical separation of the two catalytic residues (with the second not in a position to act as a nucleophile itself) has led to confusion in some literature that GH18 and other enzymes (notably [[GH25]]) may be inverting enzymes; this is certainly not the case for GH18 and is unlikely to be the case for [[GH25]].[[Image:mechanism.jpg|thumb|right|600px|'''Catalytic mechanism of family 18 chitinases.''' The picture shows the mechanism proposed for ChiB from ''Serratia marcescens''. Panel c shows the oxazolium ion intermediate which is to be hydrolyzed by an incoming water molecule. The picture is taken from<cite>Daan2001</cite>.]] |
| + | The D-X-E motif (Asp140-Glu144 in the Figure) is part of a diagnostic D-X-X-''D''-X-D-X-E motif that includes two more aspartates of which the one in italics (Asp140 in the Figure) is known to be essential for catalytic activity. There are several other conserved residues in the catalytic center that play important roles during catalysis, related to distortion of the -1 sugar, activation of the acetamido group and/or cycling of the p''K''a of the catalytic glutamate <cite>Synstad2004</cite>. The O6 of the –1 sugar interacts with the side chain of yet another semi-conserved aspartate (Asp215 in the Figure). In enzymes with an acidic pH optimum this residue is an asparagine. | ||
| + | |||
| + | === Catalytically inactive members === | ||
| + | One unusual of feature of plant members of the GH18 family is the large number of sequences that encode catalytically-inactive proteins that function as enzyme inhibitors or lectins. Phylogenetic analysis of the plant GH18 family reveals clear distinction between hevamine-type chitinases, putative chitinases and narbonins <cite>Durand2005</cite>. Out of the major subfamilies, only the one that contains hevamine contains enzymes of demonstrated activity <cite> TerwisschavanScheltinga1996</cite>. The subfamily of GH18 that contains xylanase inhibitor proteins (XIP) emerged from the hevamine cluster along with concanavalin B. All have non-conservative substitutions of one of the acidic amino acid residues in the catalytic region. In the structure of concanavalin B the catalytic Glu residue is replaced by Gln <cite>Henniga1995</cite>, which mostly accounts for the lack of chitinase activity reported for this protein. The XIP-type inhibitors all have the third aspartic acid DxxDxDxE mutated into an aromatic residue whereas the catalytic glutamate residue is only conserved in the prototype of cereal xylanase inhibitors, XIP-I (isolated from ''Triticum aetivum'') <cite>Juge2004</cite>. In XIP-I and narbonin, the glutamic acid residues are present in an equivalent position to the catalytic residue in hevamine, but their side chain is fully engaged in salt bridges with neighbouring arginine residues <cite>Hennigb1995 Payan2003</cite>, preventing chitinase activity despite the presence of the catalytic residue. Furthermore in both XIP-I and narbonin, the position equivalent to residue Asp in hevamine, which has been proposed to stabilize the positively charged [[oxazolinium ion]] reaction [[intermediate]] <cite>TerwisschavanScheltinga1996</cite>, is occupied by a bulky residue <cite>Hennigb1995 Payan2003</cite>. The mutation of this Asp residue in alanine in hevamine led to a mutant with approx. 2% residual activity <cite>Bokma2002</cite>. The most striking disruption of the cleft in XIP-I and narbonin is caused by the mutation of subsite -1 Gly which participates in the hydrogen-bonding network with the ligand <cite>ATVA1 TerwisschavanScheltinga1995</cite>, resulting in complete obstruction of subsite -1 and preventing access to the catalytic residue <cite>Payan2003</cite>. Xylanase inhibitors appeared after the emergence of the various subfamilies of chitinases from their common ancestor. In this respect, the xylanase inhibitors are a relatively new acquisition, and so far no protein has been reported to display both xylanase inhibition and chitinase activities. GH18 XIP-type inhibitors can inhibit xylanases from [[GH10]] and [[GH11]] families <cite>Juge2004</cite>. The inhibition specificity of the GH18 xylanase inhibitors can be explained on the basis of the solved 3-D structure of XIP-I in complex with a [[GH10]] xylanase from ''A. nidulans'' and a [[GH11]] xylanase from ''P. funiculosum'' <cite>Payan2004</cite>. | ||
== Three-dimensional structures == | == Three-dimensional structures == | ||
| − | Although these enzymes are frequently multi-modular, the catalytic domains are α / β barrels <cite>Perrakis, | + | [[Image:FIGURE ChiA_ChiB.jpg|thumb|right|500px|'''Structures of ChiA (left) and ChiB (right) from ''Serratia marcescens''.''' Panels A and B show C-alpha traces of the complete two-domain enzymes, with a substrate-binding non-catalytic domain pointing to the upper left (A) or lower right (B). The side chains of aromatic residues (possibly) involved in polysaccharide binding are shown in dark blue, whereas the side chain of the acid/base catalytic glutamate is shown in green. Panel C shows details of ChiA in complex with an chito-octamer (PDB ID[{{PDBlink}}1ehn 1ehn]) and panel D shows details of ChiB in complex with a chito-pentamer (PDB ID[{{PDBlink}}1e6n 1e6n]). Subsites are numbered. This picture was taken from <cite>Zakariassen2009</cite>.]] |
| − | + | Although these enzymes are frequently multi-modular, the catalytic domains are α / β barrels <cite>Perrakis,ATVA1</cite>. While several structures for complete bi-modular chitinases are available <cite>Perrakis,Aalten2000</cite> (see Figure), available structural information for multi-modular enzymes is often limited to the isolated catalytic domain. | |
| − | Work on the conformational itinerary of catalysis which is extremely similar to other retaining enzymes active on ''gluco''-configured substrates, was provided by the van Aalten group <cite>Daan2001</cite> in 2001 through the trapping of a distorted Michaelis complex in <sup>1,4</sup>B conformation and thus extremely similar to the <sup>1</sup>S<sub>3</sub> skew boats | + | Work on the conformational itinerary of catalysis which is extremely similar to other [[retaining]] enzymes active on ''gluco''-configured substrates, was provided by the van Aalten group <cite>Daan2001</cite> in 2001 through the trapping of a distorted Michaelis complex in <sup>1,4</sup>''B'' conformation and thus extremely similar to the <sup>1</sup>''S''<sub>3</sub> skew boats observed in [[GH5]] <cite>Davies1998</cite> for example or the <sup>4</sup>E conformation originally seen for a "neighboring group" enzyme in [[GH20]] <cite>Tews1996</cite>. More recently, a similar conformation has been observed for the Michaelis complex of another neighboring group enzyme, the [[GH84]] O-GlcNAcase <cite>He2010</cite>. Fungal GH18 enzymes are considered as possible therapeutic targets and a number of programmes are probing this area (for example <cite>Housten2002</cite>). |
| − | |||
| − | |||
| − | |||
| − | |||
== Family Firsts == | == Family Firsts == | ||
;First sterochemistry determination: Sometimes '''incorrectly''' reported as inverting, this family performs catalysis with '''retention''' of anomeric configuration as first shown on the ''Bacillus ciculans'' enzyme <cite>Armand1994</cite>. | ;First sterochemistry determination: Sometimes '''incorrectly''' reported as inverting, this family performs catalysis with '''retention''' of anomeric configuration as first shown on the ''Bacillus ciculans'' enzyme <cite>Armand1994</cite>. | ||
| − | ;First catalytic nucleophile identification: This family is one of many that uses neighbouring group participation for catalysis with the N-acetyl carbonyl group acting as the nucleophile; first proposed ( | + | ;First [[catalytic nucleophile]] identification: This family is one of many that uses neighbouring group participation for catalysis with the N-acetyl carbonyl group acting as the nucleophile; first proposed (we believe) for this family in <cite>TerwisschavanScheltinga1995</cite>. |
| − | ;First general acid/base residue identification: On the basis of 3-D structure <cite>Perrakis</cite>. | + | ;First [[general acid/base]] residue identification: On the basis of 3-D structure <cite>Perrakis</cite>. |
| − | ;First 3-D structure: The first two 3-D structures for catalytically active GH18 members were the ''Serratia marcescens'' chitinase A and the plant defence protein hevamine published "back-to-back" in ''Structure'' in 1994 <cite>Perrakis,ATVA1</cite>. In retrospect, however, the non-catalytic " | + | ;First 3-D structure: The first two 3-D structures for catalytically active GH18 members were the ''Serratia marcescens'' chitinase A and the plant defence protein hevamine published "back-to-back" in ''Structure'' in 1994 <cite>Perrakis,ATVA1</cite>. In retrospect, however, the non-catalytic "narbonin" structure was arguably the first GH18 3-D structure, although it is has no enzymatic activity <cite>Hennig1993</cite>. |
== References == | == References == | ||
| Line 60: | Line 63: | ||
#Perrakis pmid=7704527 | #Perrakis pmid=7704527 | ||
#ATVA1 pmid=7704528 | #ATVA1 pmid=7704528 | ||
| − | |||
#Armand1994 pmid=8168626 | #Armand1994 pmid=8168626 | ||
#Koshland1953 Koshland, D. (1953) Biol. Rev. 28, 416. | #Koshland1953 Koshland, D. (1953) Biol. Rev. 28, 416. | ||
| Line 80: | Line 82: | ||
#Juge2004 pmid=14871661 | #Juge2004 pmid=14871661 | ||
#Durand2005 pmid=15794761 | #Durand2005 pmid=15794761 | ||
| − | + | #TerwisschavanScheltinga1995 pmid=7495789 | |
| + | #Horn2006a pmid=16420473 | ||
| + | #Horn2006b pmid=17116887 | ||
| + | #Sorbotten2005 pmid=15654891 | ||
| + | #Varrot2003 pmid=12842048 | ||
| + | #Bokma1997 pmid=9271197 | ||
| + | #Eijsink2008 pmid=18367275 | ||
| + | #Hult2005 pmid=15717865 | ||
| + | #Aalten2000 pmid=7675786 | ||
| + | #Zakariassen2009 pmid=19244232 | ||
| + | #Synstad2004 pmid=14717693 | ||
| + | #Sakuda1987 pmid=3570982 | ||
</biblio> | </biblio> | ||
[[Category:Glycoside Hydrolase Families|GH018]] | [[Category:Glycoside Hydrolase Families|GH018]] | ||
Latest revision as of 14:17, 18 December 2021
This page has been approved by the Responsible Curator as essentially complete. CAZypedia is a living document, so further improvement of this page is still possible. If you would like to suggest an addition or correction, please contact the page's Responsible Curator directly by e-mail.
| Glycoside Hydrolase Family GH18 | |
| Clan | GH-K |
| Mechanism | retaining |
| Active site residues | known (acid/neighbouring group) |
| CAZy DB link | |
| http://www.cazy.org/GH18.html | |
Substrate specificities
Family GH18 is unusual in having glycoside hydrolases that are both catalytically active chitinases (EC 3.2.1.14) and endo-β-N-acetylglucosaminidases (EC 3.2.1.96) and also sub-families of non-hydrolytic proteins that function as carbohydrate binding modules / "lectins" or as xylanase inhibitors.
The active chitinases comprise non-processive endo-acting enzymes as well as processive enzymes with exo- and endo-binding preferences [1, 2]. Most enzymes primarily produce chitobiose, but some endo-acting family 18 chitinases are not capable of cleaving trimers or tetramers and thus yield longer products. Note that in older literature the endo-/exo-character of these enzymes often is assessed by studying the degradation of oligomeric substrates, and that several recent studies have shown this method to be invalid.
Family 18 chitinases break down all forms of chitin at varying rates depending on the enzyme and the substrate. They also act on chitosan with degrees of acetylation as low as 13 % [3] and some are known to degrade peptidoglycan [4]. Studies on family 18 chitinases from Serratia marcescens have suggested that most subsites for sugar binding show some promiscuity with the notable exception of subsite -1 [1].
Kinetics and Mechanism
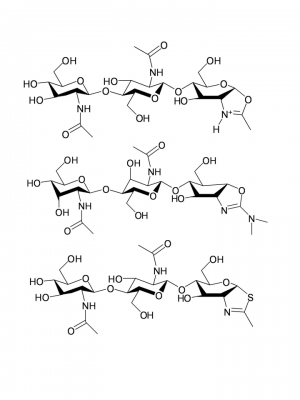
GH18 enzymes perform enzymic catalysis with retention of anomeric configuration. They belong to a group of enzymes (including GH families 18, 20, 25, 56, 84, and 85) that perform catalysis using a double-displacement reaction but instead of the more common enzyme-derived nucleophile they utilize the N-acetamido carbonyl oxygen in what is termed neighboring group participation (or substrate participation or anchimeric assistance). Figures showing such a mechanism date back to Koshland's 1953 review [5]; indeed they frequent the chemical literature of participating groups long before that, but it is primarily through the work on GH18 [6] and soon after GH20 [7, 8] that such a mechanism became well established. In such a mechanism, which occurs with (net) retention of anomeric configuration, the enzyme provides a general acid function to protonate the leaving group to facilitate its departure with the substrate carbonyl oxygen playing the role of nucleophile to generate a bicyclic "oxazolinium ion" intermediate, which subsequently breaks down following the microscopic reverse via hydrolysis or occasionally transglycosylation). Such a mechanism has a number of facets, one of which is its potential inhibition using thiazolines [9]. Allosamidin, a pseudotrisaccharide consisting of two N-acetylallosamine sugars linked to an allosamizoline moiety [10], is a well known natural compound that is a high affinity inhibitor of family 18 chitinases (see Figure).
Like in many other enzymes acting on polysaccharides the substrate-binding clefts of processive family 18 chitinases are lined with aromatic residues. Family 18 chitinases have proven very useful to gain insight into the structural basis of a processive mechanism and in the importance of such a mechanism for biomass-converting efficiency [11, 12]. As previously suggested for cellulases in e.g. [13], aromatic residues close to the catalytic center play a crucial role in processivity [14].
Catalytic Residues
The catalytically-active GH18 enzymes use a double displacement reaction mechanism with neighboring group participation(see Figure). In this mechanism the carbonyl oxygen of the substrate acts as a nucleophile, with assistance from a carboxylate (Asp) that acts to deprotonate the N-acetamido nitrogen during oxazolinium ion formation/breakdown. A second catalytic acid residue (glutamate in family GH18, but often also Asp in other families using this mechanism) acts as a general acid/base to protonate the glycosidic oxygen to assist in the departure of the aglycon, and to deprotonate the nucleophilic water molecule during the hydrolysis of the oxazolinium ion intermediate. In family GH18 the two catalytic carboxylates are found in an D-X-E motif whereas in other families the carboxylates may be adjacent, such as the DD motif in family GH84 (for example see [15]). The physical separation of the two catalytic residues (with the second not in a position to act as a nucleophile itself) has led to confusion in some literature that GH18 and other enzymes (notably GH25) may be inverting enzymes; this is certainly not the case for GH18 and is unlikely to be the case for GH25.
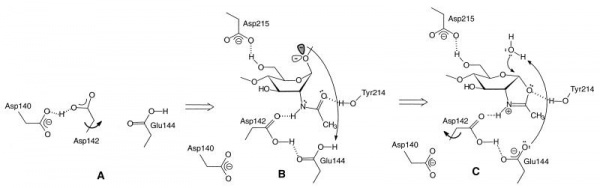
The D-X-E motif (Asp140-Glu144 in the Figure) is part of a diagnostic D-X-X-D-X-D-X-E motif that includes two more aspartates of which the one in italics (Asp140 in the Figure) is known to be essential for catalytic activity. There are several other conserved residues in the catalytic center that play important roles during catalysis, related to distortion of the -1 sugar, activation of the acetamido group and/or cycling of the pKa of the catalytic glutamate [17]. The O6 of the –1 sugar interacts with the side chain of yet another semi-conserved aspartate (Asp215 in the Figure). In enzymes with an acidic pH optimum this residue is an asparagine.
Catalytically inactive members
One unusual of feature of plant members of the GH18 family is the large number of sequences that encode catalytically-inactive proteins that function as enzyme inhibitors or lectins. Phylogenetic analysis of the plant GH18 family reveals clear distinction between hevamine-type chitinases, putative chitinases and narbonins [18]. Out of the major subfamilies, only the one that contains hevamine contains enzymes of demonstrated activity [19]. The subfamily of GH18 that contains xylanase inhibitor proteins (XIP) emerged from the hevamine cluster along with concanavalin B. All have non-conservative substitutions of one of the acidic amino acid residues in the catalytic region. In the structure of concanavalin B the catalytic Glu residue is replaced by Gln [20], which mostly accounts for the lack of chitinase activity reported for this protein. The XIP-type inhibitors all have the third aspartic acid DxxDxDxE mutated into an aromatic residue whereas the catalytic glutamate residue is only conserved in the prototype of cereal xylanase inhibitors, XIP-I (isolated from Triticum aetivum) [21]. In XIP-I and narbonin, the glutamic acid residues are present in an equivalent position to the catalytic residue in hevamine, but their side chain is fully engaged in salt bridges with neighbouring arginine residues [22, 23], preventing chitinase activity despite the presence of the catalytic residue. Furthermore in both XIP-I and narbonin, the position equivalent to residue Asp in hevamine, which has been proposed to stabilize the positively charged oxazolinium ion reaction intermediate [19], is occupied by a bulky residue [22, 23]. The mutation of this Asp residue in alanine in hevamine led to a mutant with approx. 2% residual activity [24]. The most striking disruption of the cleft in XIP-I and narbonin is caused by the mutation of subsite -1 Gly which participates in the hydrogen-bonding network with the ligand [6, 25], resulting in complete obstruction of subsite -1 and preventing access to the catalytic residue [23]. Xylanase inhibitors appeared after the emergence of the various subfamilies of chitinases from their common ancestor. In this respect, the xylanase inhibitors are a relatively new acquisition, and so far no protein has been reported to display both xylanase inhibition and chitinase activities. GH18 XIP-type inhibitors can inhibit xylanases from GH10 and GH11 families [21]. The inhibition specificity of the GH18 xylanase inhibitors can be explained on the basis of the solved 3-D structure of XIP-I in complex with a GH10 xylanase from A. nidulans and a GH11 xylanase from P. funiculosum [26].
Three-dimensional structures
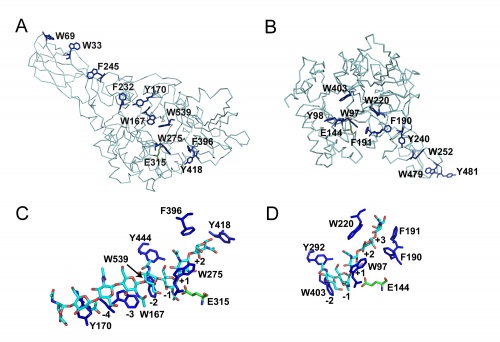
Although these enzymes are frequently multi-modular, the catalytic domains are α / β barrels [25, 27]. While several structures for complete bi-modular chitinases are available [27, 28] (see Figure), available structural information for multi-modular enzymes is often limited to the isolated catalytic domain. Work on the conformational itinerary of catalysis which is extremely similar to other retaining enzymes active on gluco-configured substrates, was provided by the van Aalten group [16] in 2001 through the trapping of a distorted Michaelis complex in 1,4B conformation and thus extremely similar to the 1S3 skew boats observed in GH5 [29] for example or the 4E conformation originally seen for a "neighboring group" enzyme in GH20 [7]. More recently, a similar conformation has been observed for the Michaelis complex of another neighboring group enzyme, the GH84 O-GlcNAcase [15]. Fungal GH18 enzymes are considered as possible therapeutic targets and a number of programmes are probing this area (for example [30]).
Family Firsts
- First sterochemistry determination
- Sometimes incorrectly reported as inverting, this family performs catalysis with retention of anomeric configuration as first shown on the Bacillus ciculans enzyme [31].
- First catalytic nucleophile identification
- This family is one of many that uses neighbouring group participation for catalysis with the N-acetyl carbonyl group acting as the nucleophile; first proposed (we believe) for this family in [6].
- First general acid/base residue identification
- On the basis of 3-D structure [27].
- First 3-D structure
- The first two 3-D structures for catalytically active GH18 members were the Serratia marcescens chitinase A and the plant defence protein hevamine published "back-to-back" in Structure in 1994 [25, 27]. In retrospect, however, the non-catalytic "narbonin" structure was arguably the first GH18 3-D structure, although it is has no enzymatic activity [32].
References
Error fetching PMID 7704528:
Error fetching PMID 8168626:
Error fetching PMID 12093900:
Error fetching PMID 11481469:
Error fetching PMID 20209544:
Error fetching PMID 20067256:
Error fetching PMID 9718293:
Error fetching PMID 12775711:
Error fetching PMID 7490746:
Error fetching PMID 1628747:
Error fetching PMID 15299319:
Error fetching PMID 8831791:
Error fetching PMID 11846790:
Error fetching PMID 12617724:
Error fetching PMID 15181003:
Error fetching PMID 14871661:
Error fetching PMID 15794761:
Error fetching PMID 7495789:
Error fetching PMID 16420473:
Error fetching PMID 17116887:
Error fetching PMID 15654891:
Error fetching PMID 12842048:
Error fetching PMID 9271197:
Error fetching PMID 18367275:
Error fetching PMID 15717865:
Error fetching PMID 7675786:
Error fetching PMID 19244232:
Error fetching PMID 14717693:
Error fetching PMID 3570982:
- Error fetching PMID 16420473:
- Error fetching PMID 15717865:
- Error fetching PMID 15654891:
- Error fetching PMID 9271197:
-
Koshland, D. (1953) Biol. Rev. 28, 416.
- Error fetching PMID 7495789:
- Tews I, Perrakis A, Oppenheim A, Dauter Z, Wilson KS, and Vorgias CE. (1996). Bacterial chitobiase structure provides insight into catalytic mechanism and the basis of Tay-Sachs disease. Nat Struct Biol. 1996;3(7):638-48. DOI:10.1038/nsb0796-638 |
- Drouillard S, Armand S, Davies GJ, Vorgias CE, and Henrissat B. (1997). Serratia marcescens chitobiase is a retaining glycosidase utilizing substrate acetamido group participation. Biochem J. 1997;328 ( Pt 3)(Pt 3):945-9. DOI:10.1042/bj3280945 |
- Error fetching PMID 20209544:
- Error fetching PMID 3570982:
- Error fetching PMID 18367275:
- Error fetching PMID 19244232:
- Error fetching PMID 12842048:
- Error fetching PMID 17116887:
- Error fetching PMID 20067256:
- Error fetching PMID 11481469:
- Error fetching PMID 14717693:
- Error fetching PMID 15794761:
- Error fetching PMID 8831791:
- Error fetching PMID 7490746:
- Error fetching PMID 14871661:
- Error fetching PMID 15299319:
- Error fetching PMID 12617724:
- Error fetching PMID 11846790:
- Error fetching PMID 7704528:
- Error fetching PMID 15181003:
- Error fetching PMID 7704527:
- Error fetching PMID 7675786:
- Error fetching PMID 9718293:
- Error fetching PMID 12093900:
- Error fetching PMID 8168626:
- Error fetching PMID 1628747:
- Error fetching PMID 12775711: